Does Progranulin in Microglia Protect Against Alzheimer’s?
Quick Links
Progranulin mutations predispose to frontotemporal dementia (FTD), but growing evidence suggests they can heighten the risk for Alzheimer’s disease as well. A paper in the September 28 Nature Medicine now sheds some light on the mechanism behind this. Researchers led by Li Gan at the Gladstone Institute of Neurological Disease, San Francisco, found that microglia lacking the protein gobble up fewer Aβ deposits. In AD mouse models, a dearth of microglial progranulin exacerbated amyloid plaques, while an excess helped mop them up. The findings hint that progranulin-based therapies being developed for FTD could help AD patients as well.
“The paper is very well done, and highlights the fact that progranulin could play a major role in Alzheimer’s pathogenesis,” said Mikko Hiltunen at the University of Kuopio, Finland. He was not involved in the study.
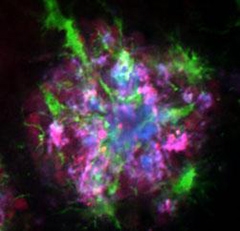
Progranulin and Plaques.
Microglia (green) surround amyloid plaques (blue), which also contain high levels of progranulin (purple). [Courtesy of S. Sakura Minami et al., Nature Medicine.]
Mutations in progranulin, a secreted growth factor, come in two flavors. Null mutations cause inherited FTD with deposits of TDP-43 protein, while missense variants that lower protein levels act as risk factors for the disease (see Jul 2006 news story; Apr 2007 conference story). Recently, geneticists have linked some of the same null and missense progranulin mutations to Alzheimer’s, especially in people who carry the Apolipoprotein E4 allele (see Apr 2013 news story; Brouwers et al., 2008; Kelley et al., 2010).
How do low progranulin levels pump up risk? Some researchers focus on progranulin’s ability to dampen neuroinflammation and activate microglia. In wild-type mice, the protein recruits microglia and stimulates phagocytosis of Aβ (see Pickford et al., 2011). In mice lacking progranulin, inflammation revs higher after injury than it does in controls (see Oct 2012 news story). And in Alzheimer’s patients and AD mouse models, progranulin expression peaks in microglia and neurons surrounding amyloid plaques, suggesting a compensatory response (see Gliebus et al., 2009; Pereson et al., 2009).
To investigate progranulin’s role in AD, Gan and first author Sakura Minami crossed progranulin knockout mice with PDAPP J9 mice, which express low levels of human amyloid precursor protein (APP) in the brain and model mild AD. While J9 mice learn normally, those without progranulin performed poorly in the Morris water maze and explored the elevated plus maze with less anxiety than littermates. Both of these behavioral changes are common in more aggressive models of AD, suggesting that a loss of progranulin exacerbated AD symptoms in the J9 animals.
Both neurons and microglia express progranulin. To dissect the contribution of the microglial version, the authors made conditional knockouts that expressed about half the normal levels of progranulin in these immune cells. They crossed these animals with PDAPP J20 mice, which express high levels of APP and develop plaques by six to seven months of age. In the crosses, plaque load tripled by seven months of age compared to J20 mice. Equal numbers of microglia gathered around plaques in the PGRN knockdowns as in controls, suggesting that the immune cells were recruited normally. However, in a phagocytosis assay, microglia isolated from the knockdowns gobbled far fewer fluorescent beads than normal microglia. The finding implies that a defect in microglial phagocytosis allowed Aβ to accumulate, the authors claim. The conditional knockouts also struggled to master the Morris water maze compared to littermate controls, even at four months of age before plaques formed.
Conversely, overexpression of progranulin in the 5xFAD mouse model, which deposits amyloid starting at two months old, lowered plaque load at five months by about one-quarter. The protein also protected hippocampal neurons from death and preserved spatial memory and fear-conditioning responses. Overexpression did not affect anxiety, which is not governed by the hippocampus. In cell cultures, microglia with excess progranulin kept neurons alive after the addition of oligomeric Aβ derived from cell lines. This finding, along with the finding that progranulin protects memory in young AD mice, implies that the growth factor lessens the toxicity of soluble Aβ, the authors speculate.
To follow up on progranulin’s effect at different ages, the authors measured levels of the protein in several different AD mouse models. Before amyloid plaques appeared, progranulin levels in brain extracts were half those of wild-type mice. RNA levels were the same, indicating no difference in gene expression. Instead, AD mice might excessively degrade or cleave the protein. In young mice, the cleavage products of progranulin, granulins, ramp up inflammation and might underlie early pathology, Gan speculated.
PGRN levels changed in older mice. As amyloid deposits grew, the protein rose in the brain, in some cases to double that of controls. Progranulin RNA levels also climbed, suggesting more gene expression. Previous studies had reported that AD patients have high brain progranulin, and the authors confirmed this, measuring twice as much protein in postmortem brain extracts from 12 AD patients as in 11 age-matched controls.
The low progranulin levels in young AD mice intrigued other researchers. “The drop in progranulin at pre-plaque ages was the most exciting thing to me,” said Samir Kumar-Singh at the University of Antwerp, Belgium. “This may be an early insult that sets the stage for increased plaque deposition and memory defects later.” Future studies should seek to confirm this and identify the mechanisms behind it, he suggested. For example, he wondered if soluble oligomers of Aβ suppress progranulin levels in some way.
Overall, this study implies that microglial, rather than neuronal, progranulin contributes the most to Alzheimer’s disease, said Aihao Ding at Weill Cornell Medical College, New York City. The finding strengthens evidence for the crucial role microglia play in modulating neurodegeneration, she noted. “Control of inflammation might hold the key for many neurodegenerative diseases,” she told Alzforum. Variants in the microglial gene TREM2 also raise risk for multiple neurodegenerative diseases (see Oct 2012 news story; Nov 2012 news story; Feb 2014 news story). Other microglial genes have been tied to Alzheimer’s, as well (see Aug 2013 news story; Sep 2013 news story).
In future work, Gan plans to look for ways to boost progranulin levels. “Enhancing progranulin could be applicable to a range of neurodegeneration patients because of its strong neuroprotective and anti-amyloid capacity,” Gan said. Researchers have identified ways to regulate progranulin expression, some of which are in clinical trials for FTD patients with progranulin mutations (for example, this Phase 2 trial of a histone deacetylase [HDAC] inhibitor and this Phase 1 study at the University of California, San Francisco). Christian Haass and colleagues at the Ludwig-Maximilians-University in Munich found that compounds that lowered the pH of cellular compartments promoted progranulin production (see Feb 2011 news story).—Madolyn Bowman Rogers
References
News Citations
- Birds of a Feather…Mutations in Tau Gene Neighbor Progranulin Cause FTD
- Salzburg: Getting to Know Progranulin, the New Heavy
- Does Progranulin Play Both Sides in AD and FTD?
- Microglial Progranulin Douses Neural Inflammation
- Mutations in TREM2 Cause Frontotemporal Dementia
- Enter the New Alzheimer’s Gene: TREM2 Variant Triples Risk
- TREM2 Variant Doubles the Risk of ALS
- Protective Microglial Gene Variant Promotes Phagocytosis
- Poor Digestion in Alzheimer’s Microglia: Is Beclin to Blame?
- Back to Basics? Boosting pH Puts Cells in Progranulin-Pumping Mode
Research Models Citations
Paper Citations
- Brouwers N, Sleegers K, Engelborghs S, Maurer-Stroh S, Gijselinck I, van der Zee J, Pickut BA, Van den Broeck M, Mattheijssens M, Peeters K, Schymkowitz J, Rousseau F, Martin JJ, Cruts M, De Deyn PP, Van Broeckhoven C. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology. 2008 Aug 26;71(9):656-64. PubMed.
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, Rademakers R, Hutton M, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, Petersen RC. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol. 2010 Feb;67(2):171-7. PubMed.
- Pickford F, Marcus J, Camargo LM, Xiao Q, Graham D, Mo JR, Burkhardt M, Kulkarni V, Crispino J, Hering H, Hutton M. Progranulin is a chemoattractant for microglia and stimulates their endocytic activity. Am J Pathol. 2011 Jan;178(1):284-95. PubMed.
- Gliebus G, Rosso A, Lippa CF. Progranulin and beta-amyloid distribution: a case report of the brain from preclinical PS-1 mutation carrier. Am J Alzheimers Dis Other Demen. 2009 Dec-2010 Jan;24(6):456-60. PubMed.
- Pereson S, Wils H, Kleinberger G, McGowan E, Vandewoestyne M, Van Broeck B, Joris G, Cuijt I, Deforce D, Hutton M, Van Broeckhoven C, Kumar-Singh S. Progranulin expression correlates with dense-core amyloid plaque burden in Alzheimer disease mouse models. J Pathol. 2009 Oct;219(2):173-81. PubMed.
External Citations
Further Reading
Papers
- Viswanathan J, Mäkinen P, Helisalmi S, Haapasalo A, Soininen H, Hiltunen M. An association study between granulin gene polymorphisms and Alzheimer's disease in Finnish population. Am J Med Genet B Neuropsychiatr Genet. 2009 Jul 5;150B(5):747-50. PubMed.
News
- Can Epigenetics Explain Variable Progranulin Expression?
- FTD Risk Factor Confirmed, Alters Progranulin Pathways
- Systems Biology Approaches Get Wnt of Progranulin’s Role in FTD
- Back to Basics? Boosting pH Puts Cells in Progranulin-Pumping Mode
- San Diego: Progranulin, Wnt, and Frizzled, Frazzle Neurons in FTD
- Genetics of FTD: New Gene, PGRN Variety, and a Bit of FUS
- Meet Progranulin, The Biomarker—A Simpler Story?
- Progranulin Controls Cutting of Inclusion Protein
- TREM2 Mystery: Altered Microglia, No Effect on Plaques
- Soothing Neuroinflammation Quells Plaques in Mice
- Progranulin—Curbs Phagocyte Appetites, Protects Neurons?
Primary Papers
- Minami SS, Min SW, Krabbe G, Wang C, Zhou Y, Asgarov R, Li Y, Martens LH, Elia LP, Ward ME, Mucke L, Farese RV Jr, Gan L. Progranulin protects against amyloid β deposition and toxicity in Alzheimer's disease mouse models. Nat Med. 2014 Oct;20(10):1157-64. Epub 2014 Sep 28 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Antwerp
With several different in vivo and cell culture approaches, including lentiviral PGRN expression and LysM promoter-driven selective ablation of PGRN from microglia, the authors elegantly showed that progranulin, especially from the microglial compartment, protects against Aβ toxicity and reduces amyloid plaque deposition.
While there is no good promoter available for selective ablation in all monocyte/macrophages lineages—for instance, the LysM promoter utilized in this study is not expressed in CD11c-positive monocytes and macrophages, and Grn transcripts in isolated CD11b+ cells were only reduced by half—the authors managed to show a threefold increase in plaque deposition kinetics accompanied by reduced retention of spatial memory. These data corroborate well with the genetic association of PGRN deficiency with increased risk of late-onset AD. While the underlying mechanism remains unidentified, decreased M1-related phagocytosis by brain microglia likely plays an important role in PGRN haploinsufficiency-related FTLD. However, an enhanced M2-phagocytosis of apoptotic neurons in Caenorhabditis elegans lacking the Grn homologue (Kao et al., 2011) also suggests that progranulin’s role in phagocytotic responses can be contextual—and requires further investigation.
Another point I found interesting was the downregulation of parenchymal progranulin in AD mice before plaque deposition in the absence of any changes in the transcript levels. While the issues of cell-bound progranulin (mostly full-length) and cell-free progranulin (full-length and proteolytic peptides) measured by a mouse ELISA employed in the study have to be sorted out first, if true, this downregulation could be an additional cause of neuronal injury in the prodromal stage of AD—and should be addressed in future studies.
References:
Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, de Luis A, Neukomm LJ, Cabello J, Farese RV, Kenyon C. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A. 2011 Mar 15;108(11):4441-6. PubMed.
View all comments by Samir Kumar-SinghMake a Comment
To make a comment you must login or register.