Do Lipids Lubricate ApoE's Part in Alzheimer Mechanisms?
Quick Links
Scientists continue to chip away at APOE4’s secrets. At the Alzheimer's Association's virtual conference on APOE and Immunity, held October 18-21, presenters focused on how this major risk factor for late-onset Alzheimer’s disease hampers lipid metabolism in glial cells, and how this problem, in turn, influences Aβ production and plaque deposition. Overall, they reported that astrocytes and microglia carrying APOE4 were sluggish at degrading lipids and became more inflamed than APOE3 glia. Strikingly, APOE4 and cholesterol cooperated to pump up Aβ production, leading to plaque deposition. The findings hint that improving lipid metabolism could be a therapeutic strategy.
- APOE4 glia process lipids poorly and become more inflamed under stress.
- APOE4 and cholesterol act together to boost Aβ production.
- Proteomic analysis suggests APOE4 accelerates many different aspects of AD.
Meanwhile, Betty Tijms of Vrije University, Amsterdam, took a broader look at APOE4, investigating how this allele alters the cerebrospinal fluid proteome along the course of AD. She turned up evidence that APOE4 accelerates not just amyloid pathology, but other disease processes as well. “I think this is so exciting,” Henrik Zetterberg of the University of Gothenburg, Sweden, said at the conference. “There is definitely an effect of APOE4 that goes beyond amyloid, and might even predate amyloid.”
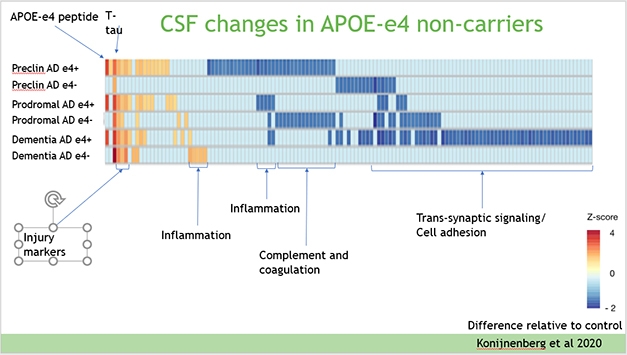
Faster Course. CSF proteins in APOE4 carriers and noncarriers follow different patterns over the course of Alzheimer’s disease. In general, the disease process appears to be faster in carriers. [Courtesy of Betty Tijms.]
APOE4 Glia Fumble Lipid Handling
APOE is an apolipoprotein, tasked with ferrying cholesterol and other lipids between cells. In the brain, it is produced mainly by astrocytes and activated microglia. Recent work by Julia TCW and Alison Goate at Icahn School of Medicine, Mount Sinai, New York, found the E4 allele disrupts the lipid balance in astrocytes and microglia derived from human iPSCs, boosting cholesterol production while hobbling its export (Jul 2018 news; Apr 2019 conference news). This imbalance allows lipid droplets to build up inside glia, and triggers inflammation (Aug 2019 news; Mar 2021 news).
Saima Machlovi in Goate’s group followed up on these data using knock-in mice that express human APOE isoforms. At the conference, Machlovi reported that cultured microglia from APOE4 knock-in mice accumulated more lipids than did APOE3 microglia, and also phagocytosed more material. APOE4 microglia cranked up their expression of cellular stress response genes, suggesting the lipid pileup put a strain on them.
Does this imbalance affect how glia react to brain injury or disease? To investigate this, Na Wang, working with Guojun Bu at the Mayo Clinic in Jacksonville, Florida, fed the demyelinating drug cuprizone to 2-month-old APOE knock-in mice for four weeks, then examined their brains a month later. Mice carrying the protective human APOE2 allele fared best, as their microglia efficiently mopped up and broke down the myelin debris. In contrast, mice carrying the APOE4 risk variant barely cleaned up the mess. Their microglia were less activated, as seen by smaller size and number, weaker Iba1 staining, and lower expression of inflammatory factors. These torpid microglia could not fully digest myelin fragments, instead storing their remains as lipid droplets. APOE3 mice landed in between these extremes. The results reinforce the idea that APOE4 microglia are less able to deal with lipid stress.
Not everyone reported such a stark difference between APOE isoforms, however. Gilbert di Paolo's group at Denali Therapeutics, South San Francisco, fed cuprizone to 3-month-old wild-type, APOE knockout, and APOE3 and -4 knock-in mice for 12 weeks and studied the ensuing mayhem in situ. Di Paolo presented these results at the conference, but told Alzforum that APOE2 data are currently being analyzed, for a fuller comparison across alleles.
The knockouts had the most dramatic phenotype. Unable to clean up the deteriorating myelin, their microglia stored the excess lipids in their cell bodies as cholesteryl esters. Di Paolo noted that this is similar to how TREM2 knockouts respond to demyelination (Nugent et al., 2020). However, the presence of either APOE3 or APOE4 prevented cholesteryl esters. Di Paolo saw only subtle differences in lipid metabolism between the isoforms. E4 microglia accumulated more plasmalogens and ceramides than E3s, and E4 astrocytes had fewer sphingolipids than their E3 counterparts.
“We suspect the microglia dysfunction may arise in the presence of amyloid and tau pathology,” di Paolo said. Another possibility is that APOE4’s effects on lipid metabolism are more pronounced in human than mouse brain. TCW, now at Boston University, likewise previously reported only mild glial differences between different knock-in mice (TCW et al., 2019).
APOE4 and Amyloid: More Production, Less Degradation
Speaking of amyloid, how do APOE isoforms affect the glial response to this stressor? Radosveta Koldamova of the University of Pittsburgh tackled this question. She injected each isoform along with synthetic Aβ42 oligomers into the brains of transgenic mice whose microglia fluoresce. A day later, microglia in mice that received ApoE3 had gobbled up almost twice as much Aβ as those that received ApoE4. Two-photon microscopy of these mice revealed that E3 caused microglia to extend processes toward Aβ and encapsulate the peptide more robustly than did E4. In keeping with this, microglia exposed to ApoE3 were more transcriptionally activated than those given E4. Notably, on a TREM2 knockout background, ApoE3 still permitted an effective microglial response to Aβ, whereas ApoE4 did not (Fitz et al., 2021).
Not only does ApoE4 protein blunt the microglial breaking of Aβ, it may also boost its making. Hao Wang, working with Scott Hansen at the Scripps Research Institute in Jupiter, Florida, cultured neurons and astrocytes from wild-type and APOE knockout mice, then examined them with super-resolution microscopy. When the scientists added conditioned media from wild-type astrocytes to wild-type neurons, they saw ApoE and cholesterol in the media together escort amyloid precursor protein (APP) into lipid rafts in the neuronal membrane. There, APP was snipped by β- and γ-secretases to make Aβ. ApoE4 elevated the amount of APP in these lipid rafts by about half, enhancing Aβ generation over that produced by ApoE3. The findings agree with a previous report that ApoE4 amplifies Aβ production compared to ApoE3 (Jun 2018 news).
On the other hand, Wang found that eliminating either cholesterol or ApoE slashed the amount of APP associated with lipid rafts to nearly zero. This greatly altered AD pathology; year-old 3xTg mice lacking cholesterol had virtually no plaques or phosphorylated tau, in contrast to robust accumulation in those with cholesterol (Wang et al., 2021).
These data add heft to the idea that lowering ApoE could squelch AD pathology. This is being explored as a therapeutic strategy by David Holtzman at Washington University School of Medicine in St. Louis and others (Apr 2018 news; Feb 2021 news).
At the conference, Chao Wang and Thomas Mahan in Holtzman’s group offered more evidence supporting this approach. They generated conditional APOE4 knock-in mice that lose APOE expression only in astrocytes when given tamoxifen, and bred these mice with the APPPS1 amyloidosis model. When the crosses were 4 months old, the scientists shut down astrocyte APOE and injected methoxy to label existing plaques. When they assessed the mouse brains two months later, existing plaques had shrunk by about half in most brain regions, and about half as many new plaques had formed compared to APPPS1 mice that still had their astrocytic APOE. The improvements seemed to be due to the glial response, as astrocytes and microglia in these mice had engulfed almost twice as much plaque material as those in controls. These phagocytic glia also had a less reactive, less inflamed phenotype, as seen by gene expression profiles.
APOE4’s Lipid Mess Sparks Inflammation
Does all this mouse and cell culture data translate to human brain? To answer this, Brandon Ebright, working with Hussein Yassine at the University of Southern California, Los Angeles, analyzed lipid changes in 21 postmortem AD brains compared to 21 age-matched control brains from the Religious Orders Study. He found that APOE4 AD brains contained more pro-inflammatory lipids, such as prostaglandin-D2 and leukotriene B4, than did APOE3 AD brains. Both had more than healthy controls. Curiously, APOE4 AD brains also contained more lipid mediators such as lipoxins and resolvins, which help soothe inflammation. Together, the data suggest APOE4 exacerbates the inflammatory response in AD brain, Ebright said.
Are there ways to restore the lipid metabolism in glial cells? Anna Podlesny-Drabiniok in Goate’s group presented one method. Using human iPSC lines from APOE3/3 and 4/4 donors to generate microglia, she found that small molecules that activate liver X receptors (LXRs) on microglia boosted cholesterol efflux and restored lysosomal function in E4 microglia.
Does APOE4 Speed All Aspects of Alzheimer's?
A body of animal model research has established that APOE4’s effects are wide-ranging, altering not just amyloid, lipids, and inflammation, but also phosphorylated tau. To look for such effects over the course of AD, Tijms analyzed cross-sectional proteomic data from ADNI CSF samples taken from 126 amyloid-positive APOE4 carriers and 67 amyloid-positive noncarriers. These data were compared with the proteomic CSF profiles of 60 amyloid-negative, cognitively healthy noncarrier controls. The data came from several sources, with 142 proteins measured by mass spectrometry (Spellman et al., 2015), another 159 by the commercial Human DiscoveryMap immunoassay, plus a dozen of the usual suspects, such as Aβ, tau, α-synuclein, via ELISA.
When Tijms stratified the data by disease stage, she found that the protein profiles of APOE3 and APOE4 carriers were distinctly different as their respective development of Alzheimer’s disease progressed (see image above). E4 carriers at the preclinical stage expressed many injury markers, but less of the inflammatory and complement proteins than did the controls. As their disease advanced toward symptoms, complement and inflammatory proteins rose to control levels, while synaptic signaling and cell adhesion proteins fell away. This might have something to do with cell loss, Tijms noted. E3 carriers, on the other hand, started their preclinical stage with decreased synaptic signaling and cell adhesion proteins. As they became prodromal, injury markers came up, as did complement. At the dementia stage, inflammatory markers rose in the E3s, but not the E4s (Konijnenberg et al., 2020).
Overall, the E4 allele accelerates AD pathogenesis, Tijms concluded. “In both carriers and noncarriers, similar processes are involved, and it seems the APOE4 allele mostly determines when a particular process becomes involved,” she said. APOE4 was already well-known to speed amyloid deposition; half of APOE4 carriers become amyloid-positive before the age of 60, Tijms noted. Next, she will analyze the lipidome of ADNI CSF samples to determine how it changes with disease progression and APOE genotype. Lipid-based studies of APOE appear poised to be the wave of the future.—Madolyn Bowman Rogers
References
News Citations
- ApoE Has Hand in Alzheimer’s Beyond Aβ, Beyond the Brain
- Could Greasing the Wheels of Lipid Processing Treat Alzheimer’s?
- ApoE4 Glia Bungle Lipid Processing, Mess with the Matrisome
- Droplets of Unsaturated Fats Burden Human ApoE4 Astrocytes
- In Human Neurons, ApoE4 Promotes Aβ Production and Tau Phosphorylation
- Human ApoE Antibody Nips Mouse Amyloid in the Bud
- Would ApoE Make a Better Therapeutic Target Than Aβ?
Research Models Citations
Paper Citations
- Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PC, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, Chen H, Srivastava A, Poda SB, Sanchez PE, Watts RJ, Sandmann T, Astarita G, Lewcock JW, Monroe KM, Di Paolo G. TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron. 2020 Mar 4;105(5):837-854.e9. Epub 2020 Jan 2 PubMed.
- Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, Sikora E, Andrews EJ, Martini AC, Karch CM, Head E, Holtzman DM, Zhang B, Wang M, Maxfield FR, Poon WW, Goate AM. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022 Jun 23;185(13):2213-2233.e25. PubMed. BioRxiv.
- Fitz NF, Nam KN, Wolfe CM, Letronne F, Playso BE, Iordanova BE, Kozai TD, Biedrzycki RJ, Kagan VE, Tyurina YY, Han X, Lefterov I, Koldamova R. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer's disease. Nat Commun. 2021 Jun 7;12(1):3416. PubMed.
- Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci U S A. 2021 Aug 17;118(33) PubMed.
- Spellman DS, Wildsmith KR, Honigberg LA, Tuefferd M, Baker D, Raghavan N, Nairn AC, Croteau P, Schirm M, Allard R, Lamontagne J, Chelsky D, Hoffmann S, Potter WZ, Alzheimer's Disease Neuroimaging Initiative, The Foundation for NIH (FNIH) Biomarkers Consortium CSF Proteomics Project Team. Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer's Disease Neuroimaging Initiative (ADNI) CSF. Proteomics Clin Appl. 2015 Aug;9(7-8):715-31. Epub 2015 Apr 24 PubMed.
- Konijnenberg E, Tijms BM, Gobom J, Dobricic V, Bos I, Vos S, Tsolaki M, Verhey F, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Frölich L, Lovestone S, Streffer J, Bertram L, Blennow K, Teunissen CE, Veerhuis R, Smit AB, Scheltens P, Zetterberg H, Visser PJ. APOE ε4 genotype-dependent cerebrospinal fluid proteomic signatures in Alzheimer's disease. Alzheimers Res Ther. 2020 May 27;12(1):65. PubMed.
External Citations
Further Reading
News
- Even In the Healthy, ApoE4 Stirs Up Trouble, Scientists Say
- Could Juicing Up Trafficking Abolish ApoE4’s Alzheimer’s Risk?
- Taming ApoE Via the LDL Receptor Calms Microglia, Slows Degeneration
- Squelching ApoE in Astrocytes of Tau-Ravaged Mice Dampens Degeneration
- WAM! New Microglial Subtype Mops Up Moribund Myelin
- In Astrocytes, ApoE4 Bungles Endocytosis, PICALM Picks Up the Slack
- ApoE Variants Modulate Astrocyte Appetite for Synapses
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
- ApoE Risk Explained? Isoform-Dependent Boost in APP Expression Uncovered
- Protective APOE3 Variant Binds More Lipids, Self-Aggregates Less
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.