ApoE4 Glia Bungle Lipid Processing, Mess with the Matrisome
Quick Links
ApoE4 predisposes people to Alzheimer’s disease by modulating astrocytes and microglia, suggest researchers led by Julia TCW and Alison Goate at the Icahn School of Medicine at Mount Sinai, New York. In a preprint on bioRχiv, the researchers describe transcriptional differences between iPSC-derived human astrocytes and microglia that express ApoE4/4 or ApoE3/3. The ApoE4/4 glia generated more cholesterol than their E3/3 counterparts. They exported and degraded it poorly, causing lipid to build up inside them. The E4/4 glia also pumped out greater amounts of proinflammatory cytokines and extracellular matrix proteins than E3/3s.
- ApoE4 astrocytes accumulate cholesterol because production and export are unbalanced
- E4 astrocytes and microglia make more extracellular matrix and inflammatory proteins than do E3 cells
- This glial phenotype is also seen in AD brain, regardless of ApoE status
Does this have anything to do with Alzheimer’s? Lo and behold, in AD brains, astrocytes and microglia behaved quite similarly to these ApoE4/4 glia. They accumulated lipid and ratcheted up inflammation. Importantly, they did so regardless of their ApoE genotype. To TCW, the data imply that ApoE4 may nudge microglia and astrocytes toward an Alzheimer's-like state. Perhaps faulty lipid metabolism is one of the earliest changes on the path to Alzheimer’s. If so, restoring glial lipid regulation could be a therapeutic approach, she suggested. The paper is under review at Cell (Sneak Peek).
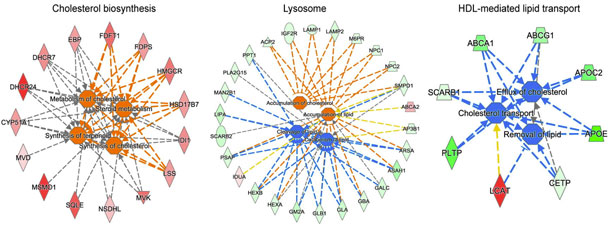
Production Up, Disposal Down. Genes for making cholesterol are expressed more in cultured human ApoE4 than ApoE3 microglia (red, orange), while genes for exporting and breaking cholesterol are mostly lower (blue, green). [Courtesy of Julia TCW.]
Other scientists agreed that the data shed light on how ApoE4 may push the brain toward disease. “This unbiased work demonstrates that in CNS-relevant glial cells, cholesterol metabolism likely represents a major ApoE4-related pathobiology in Alzheimer’s and aging brains,” Guojun Bu at the Mayo Clinic in Jacksonville, Florida, wrote to Alzforum (full comment below). Shane Liddelow at New York University was impressed by how comprehensive the analyses were. “This is a great paper. It opens up a whole new avenue of hypotheses for us to investigate,” he said.
TCW and others previously reported that ApoE4 prods microglia and astrocytes to crank up inflammation in response to external stressors (Apr 2017 conference news). At recent meetings, TCW laid out problems with lipid metabolism in ApoE4 glia (Jul 2018 conference news; Apr 2019 conference news).
In the new paper, the scientists elaborate. They generated 13 iPSC lines: six from people homozygous for ApoE4/4, the remainder from people with the ApoE3/3 genotype. They differentiated each line into pure cultures of microglia or astrocytes, and analyzed gene expression. ApoE4/4 cells, regardless of type, more highly expressed genes involved in the synthesis of cholesterol and steroids than did ApoE3/3 cells. At the same time, ApoE4/4 microglia expressed fewer Liver X and retinoid-X receptors and ligands. LXRs and RXRs are transcription factors that bind lipids and switch on expression of lipid exporters such as ApoE.
Because LXRs and RXRs are lipid sensors, the finding suggested that E4/4 glia might have trouble sensing and regulating their lipid levels. In keeping with this, E4/4 astrocytes made less ApoE and ABCA1 than did ApoE3/3 cells. These proteins help ferry cholesterol from the cell. Meanwhile, ApoE4/4 microglia expressed less LAMP1, a lysosomal protein that binds cholesterol and facilitates its breakdown. Overall, the findings depicted an imbalance between cholesterol production and disposal in E4/4 glia (see image above).
If true, then cholesterol levels should rise in these cells. The authors confirmed this using gas chromatography and mass spectrometry in ApoE4/4 and ApoE3/3 isogenic lines. (To make sure whatever effect was observed was due to ApoE genotype and nothing else, the scientists picked two E4/4 lines and used CRISPR to edit the genotype to E3/3.) Astrocytes from the E4/4 parent lines contained 20 percent more cholesterol than the isogenic E3/3 lines did. This was in the form of free cholesterol, rather than cholesterol esters, and primarily accumulated in lysosomes. The data support the idea that E4/4 glia fail to properly dispose of cholesterol, and gradually become stuffed with lipid.
How, if at all, does lipid overload affect interactions with other cell types? To find out, the authors analyzed gene expression in mixed cortical cultures of neurons and astrocytes generated from human iPSCs. Compared with ApoE3/3 astrocytes, ApoE4/4 astrocytes not only turned up expression of lipid synthesis proteins, but also highly overexpressed extracellular matrix proteins. ECM proteins, collectively known as the matrisome, include structural proteins such as collagens and proteoglycans, as well as signaling proteins, including cytokines and growth factors. The highly expressed matrisome proteins in these E4/4 cultures would heighten lipid synthesis, cell migration, and the inflammatory response, the authors predicted, tying lipid dysregulation to inflammation.
Does this happen in brain? The authors analyzed RNAseq data from healthy ApoE4/4 and ApoE3/3 brains in the Mount Sinai Brain Bank as well as from the Religious Orders Study and Memory and Aging Project (ROSMAP). In multiple regions of the temporal and frontal cortex that the authors examined, ApoE4/4 astrocytes and microglia indeed expressed more ECM proteins than E3/3s did. As in culture, E4/4 astrocytes and microglia made more cholesterol than their E3/3 counterparts did, but poorly exported and degraded it.
What about Alzheimer’s disease? Astrocytes and microglia in AD brains expressed much higher levels of matrisome proteins and inflammatory cytokines than did glial cells from healthy controls. Curiously, this did not depend on the person’s ApoE genotype. In essence, glia in ApoE3/3 AD brains had a similar phenotype to glial cells in healthy ApoE4/4 brains. To the authors, this implies that ApoE4/4 brains may already be on the path to AD even while the person is outwardly healthy.
The involvement of the matrisome in Alzheimer’s is a new finding, TCW noted. While it has been extensively studied in cancer and acute injury models, the matrisome has not drawn much attention in neurodegenerative disease.
In future work, TCW wants to determine how all these glial expression changes interact, and which come first. She believes the buildup of lipids in glia may trigger both inflammatory response and matrisome upregulation. To test this idea, she will stress cultured glia with cholesterol, cytokines, or lipid debris, and see what changes downstream.
She noted that in the brain, failure to export lipids from astrocytes could starve neurons and oligodendrocytes of the raw materials needed for proper myelin maintenance (Camargo et al., 2017). If ApoE4 carriers have thinner myelin sheaths, that might leave them more vulnerable to neurodegeneration late in life, TCW speculated. Intriguingly, some evidence suggests that women who carry an ApoE4 allele lose more myelin at menopause than do ApoE3 carriers (Aug 2018 conference news).
If lipid dysregulation is the root of the problem, would restoring normal lipid metabolism help ward off Alzheimer’s? Direct activators of LXR/RXR, such as the cancer drug bexarotene, restore cholesterol homeostasis and have been tested for atherosclerosis, but their liver toxicity makes them problematic for chronic use (for review, see Fessler, 2018). Pharmaceutical companies are developing indirect modulators of LXR/RXR that appear safer (Fan et al., 2018). In collaboration with Eli Lilly and Company, TCW is testing whether indirect modulators improve lipid regulation in cultured glia.
Bexarotene lowers amyloid plaques in mouse models, but a small Phase 2 trial gave conflicting results, reporting a slight benefit only in people who did not carry the ApoE4 allele (Feb 2012 news; Feb 2016 news). No follow-up bexarotene trial has been announced. A trial of bexarotene kinetics in cerebrospinal fluid found that very little of the drug enters the central nervous system. With low nanomolar exposure in CSF, bexarotene boosted ApoE CSF levels by one-quarter, but did not alter Aβ peptides (Ghosal et al., 2016). TCW noted that her findings suggest bexarotene and other LXR/RXR activators could be given at an early disease stage before fibrillar amyloid has formed. The goal would be to restore normal glial lipid metabolism rather than solubilizing plaque.
Meanwhile, Liddelow was particularly intrigued by potential interactions between astrocytes and microglia. Microglia need exogenous cholesterol to survive, and they get most of it from astrocytes (Jun 2017 news). Without it, microglia enter an inflammatory state, Liddelow said. Because ApoE4 astrocytes poorly export cholesterol, they may contribute to microglial inflammation, he suggested. “It’s exciting, because this is a description of astrocyte dysfunction that could be driving microglial dysfunction,” Liddelow told Alzforum.
Importantly, all findings reported in this manuscript were specific to human cells. The authors purified astrocytes and microglia from transgenic mice carrying human ApoE3 and ApoE4, and analyzed their gene expression. In stark contrast to the human iPSC-derived glia, E4 and E3 mouse glia had similar lipid metabolism. However, E4 mouse cells did recapitulate the spike in ECM and inflammatory pathways seen in human cultures and brain samples. This highlights the importance of using human iPSC models to study human disease, TCW noted.—Madolyn Bowman Rogers
References
News Citations
- ApoE and Tau: Unholy Alliance Spawns Neurodegeneration
- ApoE Has Hand in Alzheimer’s Beyond Aβ, Beyond the Brain
- Could Greasing the Wheels of Lipid Processing Treat Alzheimer’s?
- Do Brain Changes at Menopause Make Women More Prone to Alzheimer’s?
- Upping Brain ApoE, Drug Treats Alzheimer's Mice
- Bexarotene—First Clinical Results Highlight Contradictions
- What Makes a Microglia? Tales from the Transcriptome
Therapeutics Citations
Paper Citations
- Camargo N, Goudriaan A, van Deijk AF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA, Dijkhuizen RM, Mansvelder HD, Chrast R, Smit AB, Verheijen MH. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017 May;15(5):e1002605. Epub 2017 May 26 PubMed.
- Fessler MB. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol Ther. 2018 Jan;181:1-12. Epub 2017 Jul 16 PubMed.
- Fan J, Zhao RQ, Parro C, Zhao W, Chou HY, Robert J, Deeb TZ, Raynoschek C, Barichievy S, Engkvist O, Maresca M, Hicks R, Meuller J, Moss SJ, Brandon NJ, Wood MW, Kulic I, Wellington CL. Small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway. J Lipid Res. 2018 May;59(5):830-842. Epub 2018 Mar 21 PubMed.
- Ghosal K, Haag M, Verghese PB, West T, Veenstra T, Braunstein JB, Bateman RJ, Holtzman DM, Landreth GE. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement (N Y). 2016 Jun;2(2):110-120. Epub 2016 Jun 17 PubMed.
External Citations
Further Reading
Primary Papers
- Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, Sikora E, Andrews EJ, Martini AC, Karch CM, Head E, Holtzman DM, Zhang B, Wang M, Maxfield FR, Poon WW, Goate AM. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022 Jun 23;185(13):2213-2233.e25. PubMed. BioRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
Hong Kong University of Science & Technology
This is an exciting study demonstrating that APOE4 is associated with dysregulation of cholesterol homeostasis in human but not mouse astrocytes and microglia. Although such an association was implicated in previous studies, including those addressing APOE genotype effects in peripheral cells such as macrophages, this unbiased work demonstrates that in CNS-relevant glial cells, cholesterol metabolism likely represents a major APOE4-related pathobiology in Alzheimer’s and aging brains.
It is also interesting that the defective cholesterol metabolism is unique to human cells. However, this observation should be interpreted with caution. In particular, it is known that primary cultured microglial cells behave very differently from when present in vivo. Other factors that could also impact the results, such as aging and sex, are difficult to be modeled and factored into consideration using cell culture systems.
Future studies using, for example, single cell/single nucleus RNA sequencing in animal and human brains across different APOE genotype, age and sex with or without Alzheimer-related pathologies, might offer more definitive answers in terms of relevance to humans and in-vivo environments.
Gladstone Institute of Neurological Disease, UCSF
This is an interesting and comprehensive whole-transcriptome study of APOE4 and Alzheimer’s disease (AD), using human iPSC-derived brain cells, mouse brain cells, and cell-type deconvoluted transcriptomic data from postmortem AD brains. An important finding is that APOE4 is associated with dysregulation of cholesterol homeostasis in human but not mouse astrocytes and microglia, suggesting a species-dependent effect of APOE4. In contrast, elevated matrisome signaling associated with chemotaxis, glial activation, and lipid biosynthesis is observed in APOE4 mixed human neuron/astrocyte culture, mouse APOE4 glial culture, and cell-type deconvoluted transcriptomic data of APOE4 glia from AD brains, suggesting that the effect of APOE4 on matrisome signaling is not species dependent.
The human-specific transcriptional effect of APOE4 stresses the importance of studying APOE4 genotype-dependent effects in human model systems. In this regard, we have shown that APOE4 is associated with increased A production in human iPSC-derived neurons, but not in mouse neurons (Wang et al., 2018), again suggesting a species-dependent effect of APOE4. With increasing evidence of species differences in various aspects of AD modeling, it seems important to test drug candidates using human iPSC-derived brain cells before moving them into clinical trials in the future.
One important question raised by this study is whether the human-specific effect of APOE4 on cholesterol homeostasis is a loss-of-function or a gain-of-detrimental-function in glial cells. It would be worth experimentally dissecting these opposite possibilities, since answering this question is crucial for potential therapeutic development based on this study.
References:
Wang C, Najm R, Xu Q, Jeong DE, Walker D, Balestra ME, Yoon SY, Yuan H, Li G, Miller ZA, Miller BL, Malloy MJ, Huang Y. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. 2018 May;24(5):647-657. Epub 2018 Apr 9 PubMed.
UT Southwestern Dallas
This great and very extensive study by Julia TCW et al. demonstrates that the involvement of APOE4 in AD pathology likely falls back to the key role of ApoE in lipid metabolism. Additionally, they discovered a novel gene set/pathway called “matrisome” in APOE4 and AD cases. These findings are of tremendous importance to the field, since they make us researchers think about why APOE isoforms contribute to coronary artery disease, myocardial infarction, and AD in the same isoform-specific stepwise pattern: APOE4>APOE3>APOE2. Whereas this work demonstrates potential drawbacks of using animal models for complex human diseases, it suggests that hiPSC-derived brain cell cultures provide a translatable in vitro model to study APOE4-dysregulated pathways in AD.
Please also check out the preLight article I wrote for this preprint: Cholesterol and matrisome pathways dysregulated in human APOE ε4 glia
I am excited to see it in the Sneak Peek with some additional data!
Picower Institute of MIT
Picower Institute for Learning and Memory
Following the posting of their preprint, Goate and colleagues have now published their work demonstrating enhanced cholesterol accumulation and decreased lipid clearance across astrocytes and microglia of APOE4 carriers. The work, which amongst its many impressive achievements examines the transcriptome of 4 cell types derived from human iPSCs across 13 individuals, uncovers a human-specific signature of dysregulated lipid metabolism that is highly variable across individual donors. How genetic heterogeneity may modify the manifestation of APOE4 phenotypes through epistatic mechanisms remains poorly understood and is of great interest to the field.
While this study exemplifies the power of employing patient-derived iPSCs to study genetic risk variants, it raises important issues regarding the variability of gene expression levels in iPS-derived cell-types across donors with broader implications for how to model GWAS variants with these technologies. Nevertheless, the work underscores the importance of cross-validation across human datasets and mouse models, and furthers our understanding of the impact of APOE4 onto lipid homeostasis and glial activation.
View all comments by Matheus VictorThe Scripps Research Institute-Florida
It’s exciting to see more studies showing the importance of lipids in biological regulation. Ample evidence suggests lipids regulate membrane protein activity. Membrane proteins, on the other hand, play key roles in many important cellular signaling processes, including neuroexcitation, inflammation, amyloid processing, viral infection, and mechanosensation, etc. Hopefully, before long, the science community will realize that lipids are one of the master regulators of cellular function.
This study shows the interaction between astrocytes and microglia, and how APOE genotype affects the transportation of lipids in the brain. From the literature, cholesterol turns out to be a key factor for many aging-related diseases; to name a few, Alzheimer's disease, cardiovascular disease and diabetes. Cholesterol signaling in the brain is mainly mediated by ApoE.
This study bring us one step forward toward understanding how cholesterol metabolism is regulated in the brain and how ApoE is involved in the process. With further study of cholesterol function and regulation, we may be able to better understand the process of aging, and hopefully, find novel ways for healthy aging.
View all comments by Hao WangAmsterdam UMC, loc. VUmc
Maastricht University; VU University Medical Centre
Even though APOE ε4 genotype is the strongest genetic risk factor for Alzheimer’s disease, the processes through which risk is increased remain largely unclear. In this study, Julia TCW and colleagues scrutinize effects of APOE e4 on transcriptomics in human induced pluripotent cell (hiPSC) models and mice. One conclusion is that APOE knockout mice do not resemble effects associated with APOE ε4 observed human beings, suggesting that mechanistic research may perhaps best focus on human cell models.
A striking finding from the "population-based" hiPSC models from seven APOE ε3ε3 carriers with mostly normal cognition, and six APOE ε4ε4 carriers with mostly AD dementia is that the cells and astrocyte-neuron co-cultures showed mostly similar transcript patterns for ε4 versus ε3 carriers—less than 1 percent of transcripts tested differed between ε4ε4 and ε3ε3.
Importantly, the authors further demonstrate that effects of APOE ε4 on a specific cell type is person-dependent by altering APOE genotype via CRIPR-CAS for single individuals, creating so-called isogenic cell lines. The authors suggest that variability between people may reflect modification of APOE ε4 effects by other risk variants near the APOE locus (i.e., haplotypes). Their functional studies further imply that APOE ε4 may impact on lipid processing in astrocytes specifically through lysosome-dependent imbalanced cholesterol synthesis and efflux and on matrisome proteins.
The study did not investigate how amyloid and tau would influence observed transcriptomic profiles or the performed functional analyses, and so it remains to be discovered how the identified processes are related to AD disease pathophysiology. Our cerebrospinal fluid proteomics study comparing effects of APOE genotype in AD patients suggested that effects of APOE may be subtle (Konijnenberg et al., 2020), and that associated biological processes, which also included the matrisome, varied with disease stage. If individual haplotypes modify APOE ε4 effects and determine which cell types are involved, it would mean that very large sample sizes are required to tease out meaningful processes for different AD subtypes.
This study contributes to the growing evidence that patients will need therapies tailored to their causes. In order to achieve this, we need to develop more sophisticated mapping of differences between people in their underlying pathophysiological processes. APOE ε4 genotype status by itself is probably insufficient to capture such complexity.
References:
Konijnenberg E, Tijms BM, Gobom J, Dobricic V, Bos I, Vos S, Tsolaki M, Verhey F, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Frölich L, Lovestone S, Streffer J, Bertram L, Blennow K, Teunissen CE, Veerhuis R, Smit AB, Scheltens P, Zetterberg H, Visser PJ. APOE ε4 genotype-dependent cerebrospinal fluid proteomic signatures in Alzheimer's disease. Alzheimers Res Ther. 2020 May 27;12(1):65. PubMed.
View all comments by Pieter Jelle VisserUniversity of Pittsburgh
This exciting new study demonstrates that APOE4 is associated with dysregulation of cholesterol homeostasis in human astrocytes and microglia. The authors used various models to prove this finding, such as human iPSC-derived brain cells, mouse brain cells, and cell-type deconvoluted transcriptomic data from postmortem AD brains.
They discovered that genes that regulate cholesterol biosynthesis were upregulated in APOE4 astrocytes and microglia in contrast to the downregulation of genes associated with intracellular cholesterol trafficking (NPC1 and NPC2) and lysosomal function. Similarly, in APOE4 carriers, there was a significant decrease in the expression of genes that regulate cholesterol efflux, such as cholesterol transporters ABCA1 and ABCA7 and apolipoproteins. This decrease was accompanied by a lower level of APOE protein and cholesterol efflux from APOE4 cells that was restored by treatment with LXR agonists to APOE3 levels. The downregulation of cholesterol efflux suggests that the level of APOE-containing HDL-like lipoproteins circulating in ISF could be decreased in APOE4, potentially affecting Aβ aggregation and clearance.
The authors predicted that the increased expression of genes that regulate cholesterol biosynthesis accompanied by the downregulation of cholesterol efflux might lead to cholesterol accumulation in APOE4 astrocytes and microglia, and this was examined by filipin staining. It will be interesting to prove this finding using more accurate methods that can quantitatively assess intracellular cholesterol accumulation, such as TOF-SIMS.
A little unexpected was the result that mouse data from APOE targeted replacement mice were discordant with the data produced using human cells. One explanation may be the use of fetal tissue in mouse experiments and the difference between cholesterol metabolism in fetal and aged brains.
Another finding was the enrichment of matrisome pathway genes, i.e., extracellular matrix glycoproteins, secreted factors, and cytokines, in APOE4 cells. The authors conclude that the enrichment of the matrisome pathway increases inflammation and promotes cholesterol synthesis that, accompanied by a decreased efflux, leads to dysregulation of cholesterol homeostasis in APOE4 carriers.
View all comments by Radosveta KoldamovaUniversity of Minnesota, Twin Cities
Despite years of intensive investigation into the role of APOE in the pathogenesis of Alzheimer’s disease, the mechanisms underlying the genetic association of the APOE4 allele with the increased risk of AD remain incompletely understood. In this comprehensive study using multiple population and isogenic lines of hiPSC-derived glial and mixed cortical models along with cell-type deconvolution of AD brain transcriptome, Julia TCW and colleagues demonstrate that APOE4 drives dysregulation of lipid metabolism in both astrocytes and microglia. While sequestration of cholesterol in lysosome escapes SREBP-mediated feedback regulation, leading to elevated cholesterol/lipid biosynthesis in APOE4 astrocytes, the crosstalk between APOE4 astrocytes and neurons causes matrisome dysregulation, triggering an inflammatory response and lipid biosynthesis, which mimics disrupted matrisome signaling in human AD brains. The study provides convincing evidence that these alterations most likely contribute to the increased AD risk associated with APOE4.
Consistent with findings from previous research, APOE4 astrocytes exhibit deficiency in APOE secretion and cholesterol/lipid efflux, and treatment with LXR agonists rescues the deficits in APOE4 astrocytes. We have shown that a unique, clinically tested HDL-mimetic peptide, 4F, enhances APOE secretion and lipidation (cholesterol/lipid efflux) in human primary astrocytes as well as mouse primary astrocytes and microglia (Chernick et al., 2018). Our preliminary studies indicate that treatment with this HDL-mimetic peptide also rescues APOE secretion and lipidation deficits in both APOE4-TR astrocytes and APOE4 hiPSC-derived astrocytes and cerebral organoids (Apr 2019 conference news; Fredriksen et al., 2021). Further experiments are underway to investigate the impact of the HDL-mimetic peptide treatment on other functional outcomes.
Intriguingly, this study reveals increased biosynthesis of terpenoid (aka isoprenoid) as well as cholesterol in APOE4 astrocytes and microglia. This is consistent with earlier findings that the levels of isoprenoids (i.e., farnesyl and geranylgeranyl pyrophosphate, FPP and GGPP), and the expression of their synthases, are elevated in AD brains (Eckert et al., 2009). The two major isoprenoids, FPP and GGPP, serve as lipid donors for an important post-translational lipid modification process of many proteins, known as protein prenylation catalyzed by prenyltransferases. The lipophilic prenyl group facilitates the anchoring of proteins in cell membranes, mediating downstream protein–protein interactions and signal transduction (Jeong et al., 2018).
Recently we have shown that protein prenylation, in particular protein farnesylation, is upregulated in human AD brains, and the genetic deletion of farnesyltransferase reduces neuropathology and rescues cognitive deficits in transgenic AD mice (Cheng et al., 2013; Jeong et al., 2021). APOE4-mediated elevation in isoprenoid biosynthesis could be another mechanism by which APOE4 increases the risk of AD.
In addition, the findings that some of the alternations in hiPSC-derived APOE4 astrocytes and microglia are not recapitulated in APOE4-TR glia have significant implications, as humanized APOE-TR/KI mice have been serving as a major approach to investigate the role of APOE in AD.
Generation of animal models that faithfully imitate human pathophysiology remains a major challenge for the research field. Whether including the human regulatory elements in the TR/KI construct improves the similarities between species awaits further investigation. While hiPSC-derived cells and organoids provide unprecedented model systems, in vivo models are indispensable as age-related systemic impacts on physiological or pathological processes can only be studied in a living organism.
References:
Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L. Farnesyl Transferase Haplodeficiency Reduces Neuropathology and Rescues Cognitive Function in a Mouse Model of Alzheimer's Disease. J Biol Chem. 2013 Oct 17; PubMed.
Chernick D, Ortiz-Valle S, Jeong A, Swaminathan SK, Kandimalla KK, Rebeck GW, Li L. High-density lipoprotein mimetic peptide 4F mitigates amyloid-β-induced inhibition of apolipoprotein E secretion and lipidation in primary astrocytes and microglia. J Neurochem. 2018 Dec;147(5):647-662. Epub 2018 Nov 26 PubMed.
Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Müller WE, Wood WG. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009 Aug;35(2):251-7. PubMed.
Fredriksen K, Vegoe A, O'brien T, Li L. Enhancing APOE lipidation in human iPSC-derived models with a clinically tested HDL mimetic peptide. 2021 Society for Neuroscience Annual Meeting 2021 Society for Neuroscience Annual Meeting. Virtual.
Jeong A, Suazo KF, Wood WG, Distefano MD, Li L. Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer's disease. Crit Rev Biochem Mol Biol. 2018 Jun;53(3):279-310. PubMed.
Jeong A, Cheng S, Zhong R, Bennett DA, Bergö MO, Li L. Protein farnesylation is upregulated in Alzheimer's human brains and neuron-specific suppression of farnesyltransferase mitigates pathogenic processes in Alzheimer's model mice. Acta Neuropathol Commun. 2021 Jul 27;9(1):129. PubMed.
View all comments by Ling LiMake a Comment
To make a comment you must login or register.