Reproducible Brain Organoids Could Offer New Models for Research
Quick Links
Brain organoids, those tiny balls of cells grown in a dish to model the human brain, are plagued by a major problem—they are each unique. That may be about to change. Scientists led by Paola Arlotta, Harvard University, Cambridge, Massachusetts, report that one particular model of the dorsal forebrain has a consistent set of cell types, with minimal variation and a developmental trajectory similar to that of the brain of the human fetus. These organoids are well suited to the study of traits related to disorders such as neurodegeneration, the authors propose in the June 5 Nature. “We may be able to, with a new level of reproducibility and certainty, establish the effect of genes and drugs relevant to disease,” Arlotta told Alzforum.
- Dorsal forebrain organoids contain cells typical of the cerebral cortex.
- Organoid-to-organoid variability is low, comparable with normal variation among human brains.
- The reproducibility is an important step toward human brain models.
“This study elegantly demonstrates that it is possible to get homogenous and reproducible brain organoids without sacrificing complexity,” wrote Doo Yeon Kim and Rudolph Tanzi, Massachusetts General Hospital, Charlestown, to Alzforum (see full comment below). “This study will largely accelerate applications of brain organoid models in drug discovery and basic mechanistic studies.”
Other researchers agreed. “The paper itself does not address Alzheimer’s or other neurodegenerative diseases, but it is an important step in establishing cerebral organoids as model systems for human disease,” wrote Juergen Knoblich, Institute of Molecular Biology, Vienna. “The potential of these in vitro models for drug discovery in many diseases, including neurodegeneration, is enormous,” he wrote to Alzforum. They may serve as a bridge between conventional animal models and human clinical trials, he added.

Neuronal Orb. In a patterned dorsal brain organoid, neurons (blue) are intermixed with astrocytes (S100B in red and GFAP in green). [Courtesy of Velasco et al., Nature.]
Years ago, the late Yoshiki Sasai of RIKEN Center for Developmental Biology, Kobe, Japan, developed a protocol for coaxing human embryonic stem cells into three-dimensional cultures of neocortical cells (Kadoshima et al., 2013). He did this by treating the stem cells with inhibitors of ROCK, Wnt, and TGF-β to guide—or pattern in organoid parlance—the cells toward cortical subtypes. He grew them in a gelatinous protein mixture which served as a matrix to support three-dimensional cultures. Around the same time, researchers led by Knoblich published an unpatterned method to create organoids, in which human stem cells or induced pluripotent stem cells (iPSCs) are placed in a support matrix sans growth factors or inhibitors (Aug 2013 news). The idea at the time was that if the cells were left to pattern themselves, they would produce a larger variety of cell types relevant to the human brain. While the latter protocol was adopted by many labs, it produced organoids with variable shapes and cell types.
“Every organoid was in a way its own snowflake; when each one is different from the next, it becomes close to impossible to use them to compare disease states to control, or the effect of drug treatments,” said Arlotta.
To see if it was possible to create organoids with more uniform shape and composition, first author Silvia Velasco and colleagues modified the Sasai protocol. They used the same trio of inhibitors but tweaked the steps to create longer-lived, healthier cultures that produced more cell types. These comprise most of the cells of the cerebral cortex, which is the region in which the scientists were most interested. They also grew self-patterned organoids according to the Knoblich protocol, and chose two other three-dimensional neuron models, patterned dorsal and patterned ventral spheroids, which they obtained using inhibitors of TGF-β and of bone morphogenetic protein (Rigamonti et al., 2016).
They grew each type in spinning bioreactor flasks, which gently agitate the cell medium and oxygenate the cells. At three and six months, they measured shape, size, and cell type in the organoids to look for consistency.
Overall, whole brain organoids had the most variable shape and size (see image below). The spheroids were a little more regular in shape, but quite small. The patterned dorsal forebrain organoids, by contrast, measured a robust 3 mm in diameter and had a uniform, round shape. Each one produced a reproducible set of dorsal forebrain-progenitor markers and an early pan-neuronal marker at first, then cortical pyramidal neuron markers later on. These markers arose no matter which stem cell line—hESC or several types of iPSC—the group used to begin with. Because of the overall consistency of these patterned dorsal forebrain organoids, the researchers focused on them for most subsequent experiments.
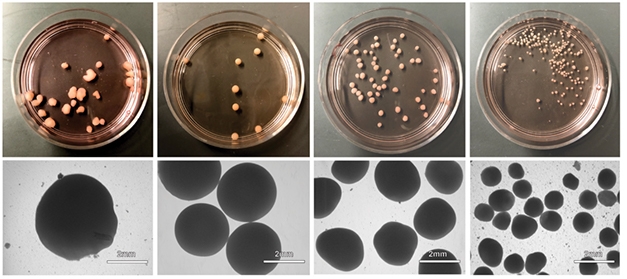
Head-to-Head Comparison. From left, whole-brain organoids had an inconsistent shape and size, dorsal forebrain organoids were uniformly round and large, while dorsal and ventral patterned spheroids were smaller and more irregular. [Courtesy of Velasco et al., Nature, 2019.]
To measure the consistency of cell type, Velasco and colleagues used high-throughput single-cell RNA-sequencing analysis to identify 166,242 cells from 21 dorsal forebrain organoids derived from five separate stem cell lines. Among the roughly 7,500 cells per organoid, she found a wide variety of cell types, including different types of progenitor, astroglia, and immature and mature neurons, all characteristic of the cerebral cortex. Importantly, each organoid made the same set of cells. Organoid-to-organoid variability was low, with proportions of individual cell types differing by about as much as they do between normal human brains.
Additionally, Arlotta said, the cells emerged in a sequence reminiscent of normal human development, starting with progenitors, then immature neurons, followed by mature ones. Within the organoids, neurons extended axons and made synapses with neighboring cells.
“This study shows that we are now headed toward the possibility of using these cells in highly standardized assays, such as might befit industry,” said Frank Yates, Sup’Biotech, Paris. “We are not there yet, but we are on the way.”
“Low variability is very important if you are looking at a disease with a subtle phenotype,” said Arnold Kriegstein, University of California, San Francisco. That said, he pointed out that although cells of organoids have transcriptional profiles similar to their native counterparts, they are not identical. He also cautioned that cells in organoids are under increased metabolic stress. “Both should be kept in mind when using organoids to model normal brain development or disease.”
While the cell types are consistent between organoids, their organization is not, Arlotta acknowledged. Cells of one type may pop up in the same general neighborhood, but these cellular regions are distributed randomly throughout the balls of cells. Future organoids will need scaffolds or some other bioengineering to separate the cells into distinct anatomical structures or cortical layers, she told Alzforum. This will allow researchers to model connectivity and answer functional questions.
Li-Huei Tsai, Massachusetts Institute of Technology, Cambridge, agreed that there was room for improvement. These organoids lack a vasculature, myelinated axons, and white-matter tracts, all relevant to neurodegenerative disease, she told Alzforum. However, this work represents an important advance in organoid research. “The most important information from this study is that there is a high correlation between the different cell types derived from organoids and fetal human cortex,” Tsai said.
These patterned dorsal organoids lack microglia, which are crucial for three-dimensional cell cultures to exhibit neurodegeneration, according to work by Kim and Tanzi (July 2018 news). Because microglia arise from a different embryological source than neurons, the organoids don’t develop them spontaneously; however, they may be relatively straightforward to add into a three-dimensional culture, Arlotta said.
Despite the caveats, dorsal forebrain organoids can be used to answer questions about which cell types are affected in a particular disease, or whether cell maturation or interactions could go awry, Arlotta told Alzforum. “The goal here is not to make brains, but to make reductionist replicas that show aspects of the development and function of the human brain that are important to understand disease,” she told Alzforum.—Gwyneth Dickey Zakaib
References
News Citations
- Mini Brain in a Dish Models Human Development
- Invading Microglia Unleash Neurodegeneration in 3D AD Culture
Paper Citations
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20284-9. Epub 2013 Nov 25 PubMed.
- Rigamonti A, Repetti GG, Sun C, Price FD, Reny DC, Rapino F, Weisinger K, Benkler C, Peterson QP, Davidow LS, Hansson EM, Rubin LL. Large-Scale Production of Mature Neurons from Human Pluripotent Stem Cells in a Three-Dimensional Suspension Culture System. Stem Cell Reports. 2016 Jun 14;6(6):993-1008. PubMed.
Further Reading
Papers
- Takebe T, Wells JM. Organoids by design. Science. 2019 Jun 7;364(6444):956-959. PubMed.
- Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Science. 2019 Jun 7;364(6444):960-965. PubMed.
Primary Papers
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019 Jun 5; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Massachusetts General Hospital
Massachusetts General Hospital
This is another groundbreaking paper from the Arlotta lab. This study tackles a fundamental challenge of brain organoid models, “heterogeneity and reproducibility.” Since generation of organoids solely depends on the self-assembly and patterning capacity of stem cells, it is inevitable to have a severe organoid-to-organoid variability if we make complex structures like a brain-in-a-dish. In this paper, Dr. Arlotta and her colleagues elegantly demonstrate that it is possible to get homogenous and reproducible brain organoids without sacrificing the complexity. This study will largely accelerate applications of brain organoid models in drug discovery and basic mechanistic studies.
Only a patterned dorsal brain organoid seems to show desirable properties out of four different brain organoid models in this study. However, it is highly likely that different types of brain organoids with similar homogeneity will follow after this study, using the similar protocol, which, we believe, will be the most significant impact of this study. In the future, we hope that the Arlotta lab and others will address another challenging but important issue—the functional homogeneity of brain organoids, which is possibly regulated by spontaneous neuronal activities and excitatory/inhibitory neural networks.
Many challenges still lie ahead in applying current brain organoid technology to study neurodegenerative diseases such as Alzheimer’s disease (AD). As shown in the new paper, the reproducible dorsal forebrain organoids exhibited a transcriptome pattern that was very similar to that of human fetal brains. Thus, fetal brain-like organoid models may be limited in recapitulating a mature/aged brain environment representative of the pathogenic cascade of AD and other neurodegenerative diseases.
We have previously shown that the expression of the adult four-repeat tau isoform (along with three-repeat tau) is essential for recapitulating AD-associated tau pathology using our three-dimensional human neural cell culture model of AD (Choi et al., 2014). Although some progress has been made, it is still highly challenging to generate homogenous brain organoids with critical brain components such as microglia, oligodendrocytes with mature myelinated axons, and vascular structures with peripheral immune components, all of which are all important to generate a comprehensive AD-in-a-dish model. Despite many challenges ahead, we are optimistic, owing to recent fast-moving innovations in organoid biology, bioinformatics, nanotechnology, and mechanical engineering, which have been instrumental in building three-dimensional human brain models in a dish.
Once again, we congratulate Dr. Arlotta and her colleagues for their very important scientific contribution to modeling a human brain in a dish.
References:
Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014 Nov 13;515(7526):274-8. Epub 2014 Oct 12 PubMed.
Make a Comment
To make a comment you must login or register.