FDA Approves a Second Amyloid Imaging Agent
Quick Links
The U.S. Food and Drug Administration on October 25 approved a second amyloid imaging agent. Flutemetamol, renamed Vizamyl, is an F18-labeled ligand developed by GE Healthcare. It joins florbetapir, or Amyvid, developed by Avid Pharmaceuticals/Eli Lilly and Company (see Apr 2012 news story). Vizamyl differs slightly from its cousin because it is approved to report the intensity of binding to amyloid plaques in false color. This could make scans easier to read, some experts agreed. The compound will be commercially available in early 2014, said a GE representative.
In regulatory terms, Vizamyl is much like Amyvid. Both are approved to indicate whether amyloid is present, and thereby support or refute an AD diagnosis. Neither is intended to diagnose AD on its own or replace other routine clinical tests for cognitive decline. As stipulated by the FDA, both require reader-training programs to instruct radiologists and other clinicians how to interpret the scans. In terms of their sensitivity and specificity, the two are roughly equivalent, said Stephen Salloway, Brown University, Providence, Rhode Island.
The main difference is that Vizamyl was approved to evaluate the density of plaques using a color-calibrated scale of signal intensity (see image below), whereas Amyvid was approved using a grayscale (see Feb 2012 news story). In reality, Salloway said, nuclear medicine physicians often use both formats to evaluate PET images. Patients and families may find it easier to appreciate abnormalities when reviewing scan results with color images with their doctor, he said. Philip Scheltens, VU University Medical Center in Amsterdam, agreed.
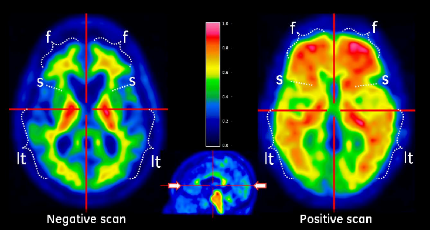
Vizamyl scans report the density of amyloid plaques using false color. Image courtesy of GE Healthcare.
Neither compound is approved to quantify amyloid plaques in the brain. That is something both companies are exploring.
Like Amyvid, Vizamyl will be made available to both neurologists and researchers. Who uses which tracer will depend on a number of factors, agreed scientists. In other countries, it may depend on which gains approval first. GE Healthcare has submitted an application for approval to the European Medicines Agency (EMA), and will apply in various other countries in the coming months and years, said the company's representative. Amyvid has already gotten EMA approval, and Lilly/Avid is seeking approval in other areas of the world. Which compound ends up being used at a given site may also depend on the proximity of production centers, said Scheltens. Both tracers rely on the F18 isotope, which has a half-life of 110 minutes and must be synthesized close to the site where it will be used. GE uses the FASTlab multitracer platform to synthetize a variety of PET ligands, including fluorodeoxyglucose. GE has 250 FASTlab sites globally. While Vizamyl is not yet approved for production on this platform, GE plans to make a Vizamyl synthesis cassette.
In recent Phase 3 brain autopsy and biopsy studies, Vizamyl showed sufficient sensitivity and specificity for Aβ brain pathology (see May 2012 conference story). Side effects were rare side and included flushing, elevated blood pressure, headache, nausea, and dizziness. A Phase 3 trial is underway for a third F18 imaging agent, florbetaben (see May 2010 news story). Agents that detect the other main pathology of Alzheimer’s disease, neurofibrillary tangles, have entered Phase 1 human testing (see Sep 2013 news story), while Navidea Biopharmaceuticals is testing another amyloid ligand in a Phase 3 trial.
While clinicians and patients have expressed enthusiasm for amyloid imaging agents, limited insurance coverage hinders widespread adoption. The Centers for Medicare and Medicaid Services (CMS) decided against reimbursing for scans in general clinical use; it will only reimburse patients for a single scan used in a clinical trial that gathers evidence about health outcomes (see Oct 2013 news story). Investigators must write a separate grant proposal to the CMS for every trial for which they want coverage of amyloid scans. Private insurers usually follow the CMS’s lead, meaning that for the time being, most patients seeking a scan will have to pay out of pocket. If evidence surfaces to suggest that the scans improve patient outcomes or disease management, the CMS will reconsider.—Gwyneth Dickey Zakaib
References
News Citations
- FDA Approves Amyvid for Clinical Use
- Miami: Can the Naked Eye Tell When a Scan Is Positive?
- Me, Too: Florbetaben, Flutemetamol Look Good in Trial
- Toronto: Sister 18F Ligands Jostle for Primacy
- Tau Tracer May Light Up All Tauopathies
Other Citations
External Citations
Further Reading
Papers
- Rinne JO, Wong DF, Wolk DA, Leinonen V, Arnold SE, Buckley C, Smith A, McLain R, Sherwin PF, Farrar G, Kailajärvi M, Grachev ID. [(18)F]Flutemetamol PET imaging and cortical biopsy histopathology for fibrillar amyloid β detection in living subjects with normal pressure hydrocephalus: pooled analysis of four studies. Acta Neuropathol. 2012 Dec;124(6):833-45. PubMed.
- Hatashita S, Yamasaki H, Suzuki Y, Tanaka K, Wakebe D, Hayakawa H. [(18)F]Flutemetamol amyloid-beta PET imaging compared with [(11)C]PIB across the spectrum of Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2013 Oct 2; PubMed.
- Vandenberghe R, Nelissen N, Salmon E, Ivanoiu A, Hasselbalch S, Andersen A, Korner A, Minthon L, Brooks DJ, Van Laere K, Dupont P. Binary classification of (18)F-flutemetamol PET using machine learning: Comparison with visual reads and structural MRI. Neuroimage. 2013 Jan 1;64:517-25. PubMed.
- Wolk DA, Grachev ID, Buckley C, Kazi H, Grady MS, Trojanowski JQ, Hamilton RH, Sherwin P, McLain R, Arnold SE. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Arch Neurol. 2011 Nov;68(11):1398-403. PubMed.
- Wolk DA, Grachev ID, Buckley C, Arnold SE. 18F-flutemetamol amyloid imaging has strong concordance with cortical biopsy histopathology. Human Amyloid Imaging 2011 Meeting Abstracts. 2011 Jan 15;
News
- Brain Imaging in Trials—How to Make It Work?
- HAI—Standardizing Amyloid PET: The Centiloid Project
- Who Will Pay for New Amyloid Scans? The Lobbying Has Begun
- Amyloid Imaging Task Force to Work Out Technology's Use
- Me, Too: Florbetaben, Flutemetamol Look Good in Trial
- Keystone: TBI—Learning From Markers, Models, and Diseases
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.