Do Microbes in the Gut Trigger Parkinson’s Disease?
Quick Links
Microbial minions teeming in the gut seem to factor in an ever-growing list of health problems, from obesity to heart disease to depression. According to a study published December 1 in Cell, microbes may also exacerbate the α-synuclein pathology that causes Parkinson’s disease (PD). Researchers led by Sarkis Mazmanian at the California Institute of Technology in Pasadena reported that in an animal model of PD, removing the normal microflora —either by raising the mice in a germ-free environment or by treating them with antibiotics—drastically reduced the severity and pushed back the onset of synuclein pathology as well as motor symptoms. Neuroinflammation, somehow escalated by short-chain fatty acids churned out by the microbes, mediated the disease cascade, the researchers proposed. What’s more, when inoculated into mice that mimic the disease, fecal bacteria taken from people with PD worsened symptoms. Taken together, the findings suggest that while gut microbes are unlikely to cause Parkinson’s by themselves, they may accelerate it.
“This is a remarkable paper showing a potential impact of microbiota in the pathogenesis of PD,” commented Marco Colonna of Washington University in St. Louis. “It is a beautiful study.”

Microbes Matter.
Typical microbiota (left panel) secrete enough SCFAs to activate microglia that can exacerbate PD, while a lack of microbes (middle panel) prevents this process. Something about microbes derived from PD patients (right panel) ramps up disease progression even more than typical microbiota. [Courtesy of Sampson et al., Cell 2016.]
From their seemingly walled-off domain in the gut, intestinal microbes have been known to influence processes that occur throughout the body, including the brain. Intense research focuses on how the microbiome exerts this striking influence from so far afield. Interestingly, more than a decade ago researchers proposed that PD could originate in the gut via infection by unknown pathogens that could spread to the brain via the vagus nerve (see Braak et al., 2003). Researchers later proposed that misfolded α-synuclein itself could travel along the gut-to-brain corridor (see Jul 2011 news).
Intestinal flora entered this picture when researchers reported that amyloidogenic proteins secreted by certain microbes could kick-start pathology in the gut and brain, perhaps by coaxing α-synuclein to misfold and oligomerize (see Oct 2016 news). Several studies have also revealed that compared with healthy controls, people with PD harbor a unique microflora with a pro-inflammatory bent, and that the relative abundance of certain microbial species even correlates with the severity of motor symptoms (see Keshavarizian et al., 2015; Scheperjans et al., 2015; Unger et al., 2016).
Given the unique complement of microbes in people with PD and the putative intestinal origins of the disease, first author Timothy Sampson and colleagues wanted to tease out the role of gut microbes in PD pathogenesis and symptoms. They used α-synuclein overexpressing (ASO) mice as models. By 12 weeks of age, these animals had a hard time on several tests of motor control and function, including balancing on beams, descending poles, removing a strip of adhesive from their noses, and freely moving their back paws when dangled upside down. By 24 weeks of age, these problems became severe. Not so in ASO mice born and raised in a strictly germ-free environment: Their performance on most motor tests equaled that of wild-type animals. Despite the known health benefits of the gut microflora, the germ-free mice did not lose weight or have weaker muscles than those with a full complement of microbes.
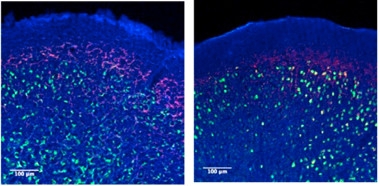
Microbes Stoke Synuclein Pathology.
ASO animals with a full complement of gut microbes (left) harbor more α-synuclein aggregates (red) in the frontal cortex than their germ-free counterparts (right). Phospho-synuclein is labelled green. [Courtesy of Sampson et al., Cell 2016.]
Intestinal microflora play a role in the neurological development of pups during gestation, hence the researchers started feeding antibiotics to ASO pups at five to six weeks of age to mimic a postnatal loss of microbiota. Just like animals born and raised in germ-free environs, the ASO mice on antibiotics performed like wild-type on motor tests at 12 and 24 weeks of age. On the other hand, colonizing germ-free ASO mice with microbes from other ASO mice vanquished this protection.
The researchers next investigated whether gut microbes hastened PD symptoms by way of exacerbating α-synuclein pathology. Compared with ASO animals with intestinal flora, those born and raised in germ-free environs had fewer α-synuclein aggregates in the caudoputamen and substantia nigra, two brain regions most affected by synuclein pathology in people with PD. Western blots of brain extracts revealed substantially less insoluble α-synuclein in germ-free ASO mice as well.
Aggregates of α-synuclein are known to boost neuroinflammation, which in turn exacerbates α-synuclein pathology, completing a vicious cycle. Recent work from Marco Prinz's at the University of Freiberg, Germany, indicates that gut microbes elevate the activation state of microglia, the brain's resident immune cells (see Jun 2015 news). In line with these previous findings, Sampson and colleagues found that microglia behaved differently depending on both the genotype of the mice and the presence of gut microbes. Microglia in the caudoputamen and substantia nigra of ASO mice appeared more mature than those in wild-type mice, in that they took on a rounder, amoeboid shape and retracted their spindly processes. However, in both germ-free ASO and germ-free wild-type animals, microglia had smaller bodies and longer processes, indicative of a resting state. Furthermore, the pro-inflammatory cytokines TNF-α and IL-6 were elevated in the brains of ASO animals replete with microbes, but not in germ-free ASO mice. These results indicated that the microbes played a hand in stoking neuroinflammation in response to elevated α-synuclein.
How do the bugs manage this mischief from the intestine? The researchers hypothesized that short-chain fatty acids (SCFAs) produced by the microbes activate the microglia, as reported by Prinz. To test this, they simply fed germ-free mice with an SCFA mixture of acetate, butyrate, and propionate. Microglia in the caudoputamen and substantia nigra of germ-free ASO mice treated with SCFAs appeared large, round, and activated, much like those in mice with microbiota.
The SCFAs also substituted for microbes in exacerbating disease pathology and motor problems: germ-free ASO mice fed with SCFAs had more α-synuclein aggregates in their brains, as well as earlier and more severe motor problems, than their untreated cousins. Treating SCFA-fed ASO animals with the anti-inflammatory drug minomycin significantly reduced TNF-a production, α-synuclein aggregation, and motor problems. Based on these findings, the researchers proposed that SCFAs produced by gut microbes ramp up neuroinflammatory responses, which in turn accelerate α-synuclein aggregation and subsequent motor dysfunction.
Role of the PD Microbiome
How does the altered microbiome observed in PD patients fit into this picture? To find out, the researchers inoculated germ-free wild-type or ASO mice with fecal bacteria from six healthy controls or six PD patients. Sequencing DNA from the animals’ droppings one, two, and three weeks later revealed that the disease status of the donor had a strong impact on the microbial communities that set up shop in the animals’ intestines. Irrespective of their genotype, animals inoculated from PD donors had microbiomes that phylogenetically grouped separately from microbiomes in mice inoculated from healthy donors. In addition, for a subset of microbial taxa, the mouse genotype also influenced their abundance, because certain microbes from the same donor thrived more in ASO than in wild-type hosts, while it was vice versa for other flora. Intriguingly, genes known to promote SCFA production were relatively elevated in microbes from PD donors, and resulted in higher proprionate and acetate and lower butyrate in mice that received PD-microbes.
The disease status of the microbe donor also affected PD symptoms in the animals. Symptoms were more severe in ASO mice colonized with bacteria from PD patients than in mice colonized with microbes from healthy donors. Wild-type mice had no change in motor function after receiving microbes from either type of donor, suggesting that the “PD-microbes” only accelerate disease in genetically predisposed animals.
The findings indicate that microbes could provide a “second hit” that unleashes disease symptoms in people who are already predisposed to PD due to genetic and/or environmental exposures, Mazmanian told Alzforum. “I don’t think the microbiome can cause motor symptoms on its own. Rather, it works in conjunction with something in the host,” he said. As exemplified by the unique microbiomes of people with PD, the communication likely goes both ways: PD-associated factors in the host alter the microbiome, which in turn exacerbates PD pathology and symptoms, he added.
“It will be important to confirm these findings in a sufficiently large cohort of PD patients,” commented Colonna. “If confirmed, this study would prompt clinical trials based on microbiota transplants.” He added that future studies should also tease out whether specific human genetic mutations associate with specific microbiota alterations.
Robert Friedland of the University of Louisville in Kentucky called the study comprehensive and impactful. He said it provided strong evidence that the microbiome plays a role in PD. However, he was surprised that the effects hinged on neuroinflammation triggered by SCFAs, as other studies reported that the fatty acids calm inflammation in the colon by supporting regulatory T cells there (see Smith et al., 2013). Mazmanian speculated that the effects of SCFAs may differ depending on the ratios of the different acids in the gut, which would mesh with the findings that microbes derived from PD patients have a unique SCFA profile. He added that it is also possible that SCFAs affect microglia, or immune cells outside of the gut, differently than they do T cells or immune cells within the gut.
Prinz said that translating the findings into a PD model was an important next step. He agreed that the fatty acids were likely to affect microglia in the brain differently than T cells in the colon. Whether SCFAs cross the blood-brain barrier and directly affect microglia or switch on the cells indirectly by activating other cells outside the brain is still unknown, Prinz said.
For his part, Friedland’s recent work suggested that amyloid proteins secreted by microbes in the gut were responsible for exacerbating the disease. He proposed that these amyloids could cross-seed α-synuclein aggregates in the gut, which could then travel from the gut to the brain. However, he added that, like SCFAs, bacterial amyloids also activate microglia. Therefore, bacterial amyloids could exacerbate PD pathology either through an inflammatory mechanism, and/or by cross-seeding α-synuclein, he speculated.
Mazmanian told Alzforum that while this study reveals a tantalizing connection between the microbiome and PD pathogenesis, researchers are still a long way from using it to help patients. Unanswered questions include how SFCAs or other microbial metabolites accelerate synucleinopathy, what makes the PD microbiome unique, and whether somehow altering it would slow the disease. If the PD microbiome accelerates disease primarily through a pro-inflammatory mechanism, it is possible that anti-inflammatory medications could help, he said. Fidgeting with specific microbes in the gut is likely to be a more complex endeavor, he added.—Jessica Shugart
References
News Citations
- Parkinson's: An Unlikely Proposal Gains Momentum
- Could Bacterial Amyloid Trigger Parkinson’s Pathology?
- To Be Hale and Hearty, Brain Microglia Need a Healthy Gut
Paper Citations
- Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003 May;110(5):517-36. PubMed.
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015 Sep;30(10):1351-60. Epub 2015 Jul 16 PubMed.
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015 Mar;30(3):350-8. Epub 2014 Dec 5 PubMed.
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016 Nov;32:66-72. Epub 2016 Aug 26 PubMed.
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013 Aug 2;341(6145):569-73. Epub 2013 Jul 4 PubMed.
Further Reading
Papers
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016 Nov 3;167(4):915-932. PubMed.
Primary Papers
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016 Dec 1;167(6):1469-1480.e12. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.