Are Reversible Amyloids Behind Liquid-Liquid Phase Separation?
Quick Links
Many proteins involved in neurodegeneration have a penchant to undergo liquid phase separation, where they coalesce into highly concentrated fluid droplets. These dynamic and reversible assemblies have important physiological functions, but they can also serve as seeding grounds for irreversible, pathogenic aggregation. Understanding the mysterious forces that bring protein droplets together, stabilize them, and let them dissolve when no longer needed has become a major focus of research. Now, researchers led by David Eisenberg, University of California at Los Angeles, have identified specific motifs that are involved. Dubbed LARKS, for low-complexity aromatic-rich kinked segments, these motifs assemble into amyloid-like protofilaments. Unlike amyloids, LARKS interact weakly, breaking apart when stirred or heated. Many different proteins bear multiple LARKS, the researchers find, suggesting that these motifs may function generally in phase separation or in the formation of protein gels or membraneless organelles. The study appeared in the February 9 Science.
- LARKS motifs in LCD domains assume amyloid-like structures.
- They may support formation of protein gels and membraneless organelles.
- This finding may expand the scope of phase-transition proteins.
Low-complexity domains (LCDs), stretches of repetitive amino acids with little apparent structure, are thought to be the driving force behind protein liquid droplets. As in the case of the ALS-associated RNA-binding protein FUS, LCDs may interact via stacked β-sheet structures found in amyloids such as Aβ. (Kato et al., 2012; Sep 2017 news).
In the new study, first author Michael Hughes confirmed that LCDs of both FUS and another RNA-binding protein linked to ALS, hnRNAP1, form reversible hydrogels, colloidal suspensions of fibril meshes in water. A technique called powder diffraction allowed Hughes to identify cross-β-sheets characteristic of amyloids in these gels. To examine atomic-level interactions that might contribute to this structure, Hughes drew on previous work implicating tyrosine motifs in gel formation. He scanned the FUS LCD for short sequences containing tandem tyrosines flanked by serine or threonine, and found two peptides that formed amyloids. Based on their sequences, he identified an additional three candidate peptides for structural studies, one in FUS, one in hnRNAP1, and one in the nucleoporin nup98, which also phase-separates. All peptides contained at least one aromatic residue, either tyrosine or phenylalanine.
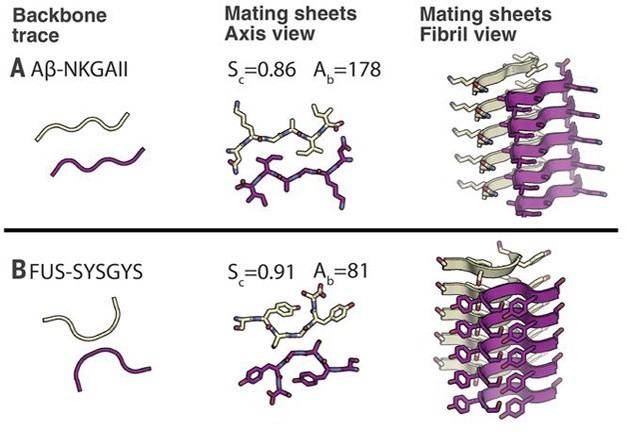
Sidelined. Unlike Aβ fibrils that hold tightly together by interactions between side chains (top panel), LARKS fibrils (bottom) are weakened by a kink that limits their interface. [Courtesy of Science/AAAS.]
Crystals of all five revealed a familiar amyloid structure. Peptide dimers stacked up and held together by hydrogen bonds [see image above]. However, while most amyloids feature a long dimer interface, these five peptides all took a sharp turn at a glycine or tyrosine residue, which limited their contact with each other, hence the “kink” in LARKS. The two stacks of kinked peptides interact much more weakly than do pathogenic amyloids. Further, interdigitated side chains, a structure termed the steric zipper, that bind some amyloids, including Aβ, are absent from LARKS filaments. Their side chains point away from the filament, leaving only weak surface interactions to hold the structure together. Computational analysis indicated that the energy required to separate these LARKS structures was only a third of that needed to separate amyloid dimers (see image below).
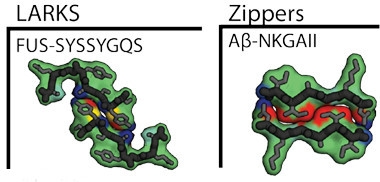
Easily Sundered.
Two FUS LARKS (left) bind more weakly than do two Aβ steric zippers (right). Red indicates stronger binding, yellow is intermediate, blue is lowest. [Courtesy of Science/AAAS.]
The low energy of adhesion suggests that numerous LARKS must cooperate in gel formation, which implies the interactions are concentration-dependent and may be transient. “If steric zippers act as molecular glue, then LARKS in LCDs act as Velcro,” the authors wrote.
The FUS LARKS sequences behaved like a full-size FUS LCD, Hughes confirmed. When he linked up the three FUS LARKS in tandem, dissolved the peptide in water and left it overnight in the cold, it formed a hydrogel. The gel completely dissolved back to the liquid state when heated to 60°C. Turning again to powder X-ray diffraction, Hughes found that the gel contained cross-β-sheets, just like gels formed by the FUS LCD.
If the role of LARKS in liquid phase transition of proteins is confirmed, this could have profound implications since LARKS motifs are common. Based on the kinked β-sheet structure, the researchers performed three-dimensional computational profiling of all 20,120 sequences in the human proteome, and identified hundreds of proteins with multiple potential LARKS sequences, many of which are known to undergo liquid-liquid phase transitions. RNA- and DNA-binding proteins accounted for one-third of the 400 proteins with the most LARKS. The authors predict that LARKS contribute to the formation of protein networks in gels and membraneless organelles.—Pat McCaffrey
References
News Citations
Paper Citations
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012 May 11;149(4):753-67. PubMed.
Further Reading
Papers
- Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017 Sep 22;357(6357) PubMed.
Primary Papers
- Hughes MP, Sawaya MR, Boyer DR, Goldschmidt L, Rodriguez JA, Cascio D, Chong L, Gonen T, Eisenberg DS. Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks. Science. 2018 Feb 9;359(6376):698-701. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.