Out of Chaos, Order: Reversible Amyloid Structure Seen in Phase Separation
Quick Links
Phase separation—the condensation of proteins with disordered, low-complexity (LC) domains into droplets—plays a central role in the pathogenesis of ALS, FTD, and other neurodegenerative diseases. On their own, LC domains appear to lack any discernible structure, freely thrashing about in solution. But get a few LC sequences together, and they readily coalesce into liquid droplets. This physiologic process serves to regulate gene expression and other critical cell functions, but in ALS/FTD, mutations in FUS and other LC domain proteins push these liquid intermediates on to pathological aggregation.
- NMR defines an amyloid core structure for FUS low-complexity domain fibrils.
- Phosphorylation at sites in fibril core disrupts phase separation by FUS-LC.
- Dynamic amyloid may contribute to LC-domain phase transitions.
What drives the phase separation of LC domains is being hotly debated in the field. Now, work from the labs of Robert Tycko, National Institutes of Health, Bethesda, Maryland, and Steven McKnight, University of Texas Southwestern Medical Center, Dallas, implicate a familiar culprit. The solid-state nuclear magnetic resonance (NMR) structure of FUS-LC domain fibrils reveals at heart an amyloid-like core. Unlike hyper-stable, pathogenic amyloids, the LC domain structure appears loosely tethered and can be disassembled by phosphorylation, which also interferes with droplet formation. This unusual, reversible amyloid offers a new organizing principle for dynamic droplet formation. The study appeared September 21 in Cell.
Bad Hair Day? In FUS-LC fibril, disordered N- and C-terminal segments flap freely around a stable amyloid core. [Courtesy of Cell, Murray et al.]
Several Alzforum commentators praised the work for its creative approach and technical difficulty. “It’s hard to figure out these structures. They were able to use solid-state NMR to obtain this really beautiful result, which is very different from other structures for amyloid fibers,” said Simon Alberti, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany.
“This is a very interesting study that will stimulate a lot of additional work in the field,” said Nicolas Fawzi, Brown University, Providence, Rhode Island.
The sequence of LC domains, which are marked by repetitious runs of a limited assortment of amino acids, suggests no particular protein structure. It bears no resemblance to known amyloid-forming peptides such as Aβ or tau, for example. Nonetheless, LC domains spontaneously self-assemble into long, unbranched amyloid-like fibrils resembling those formed by Aβ or α-synuclein.
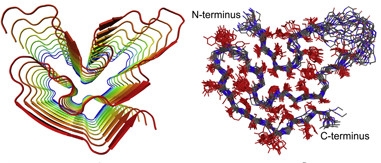
Stacking Up.
Cartoon on left shows overall fold and in-register parallel alignment of residues 37-97, forming core of fibril structure. Right: superposition of 20 low-energy models of one monomer, backbone in blue, amino acid side chains in red. Sequence stretch in top right corner is disordered. [Courtesy of Cell, Murray et al.]
To solve their structure, first authors Dylan Murray and Masato Kato analyzed fibrils formed from a 214-residue recombinant FUS-LC-domain. Most of the peptide was disordered, but about 40 residues appeared locked into place, forming the core of the fibril. With additional labeling and NMR, they identified a segment from residues 39 to 95 with a classic amyloid cross-β structure, where serpentine monomers containing short β strands aligned in parallel and perpendicular to the fiber axis. Outside the core, the tails of the protein displayed no discernable order.
The peptide backbone adopted a curve similar to other amyloids, especially α-synuclein fibrils, despite using a different repertoire of amino acids and intermolecular interactions. The FUS-LC domain lacks hydrophobic residues, which stabilize the core of pathogenic amyloid fibrils. While it’s not exactly clear how the FUS fibril core holds together, Tycko suspects a network of hydrogen bonds and dipole interactions, which are weaker than hydrophobic interactions. This may explain why FUS fibrils readily fall apart when diluted or exposed to mild detergents.
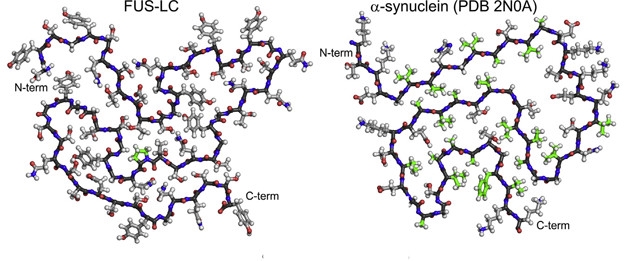
Unlikely pair. FUS-LC and α-synuclein amyloids share similar folds, despite a complete lack of sequence homology. [Courtesy of Cell, Murray et al.]
Tycko said it’s a mystery why the core forms from a segment of the LC domain that looks pretty much like every other part of the domain. “It’s kind of surprising. It could be that this particular segment is chosen because the side chains pack a little bit better than in some other segment, but that's a question for future research,” he told Alzforum.
McKnight’s group had previously found that phosphorylation of FUS-LC domains by DNA-dependent protein kinase (DNA-PK) inhibits their incorporation into hydrogels; more recently Fawzi’s lab found that phosphorylation also prevents droplet formation (Han et al., 2012; Aug 2017 news). When the investigators incubated FUS-LC domain solution with DNA-PK and ATP, they got a similar result: droplets formed transiently, and then disappeared. But was this due to disruption of the core structure, or some other effect of phosphorylation?
To answer this question, the investigators mapped the locations of 14 DNA-PK phosphorylation sites in the LC domain. They then mutated each site to alanine, and determined the effect on liquid droplet formation in the presence of DNA-PK. They discovered that only phosphorylation within the fibril core sequences destabilized droplets. Phosphorylation outside the structural core had little or no effect.
“This supports the idea that there is some kind of structure forming within the droplets, that at least involves the same core-forming segment that we’ve shown in the fibrils, and might actually involve a similar molecular structure,” Tycko said. “It’s not proof, but it's the best data we have to date.”
Of course, any cross-β structure in the droplets would involve only a few LC domain monomers, not the thousands found in a fibril, Tycko said. Molecular simulations suggested that even small clusters of FUS-LC domain can adopt metastable oligomer assemblies with the β core structure.
Party of Five. In a molecular dynamics simulation, small pentameric assemblies of FUS-LC are able to persist in solution. [Courtesy of Cell, Murray et al.]
The results will fuel an ongoing debate about how liquid protein droplets form and persist. Does defined protein structure mediate protein assembly, as McKnight has suggested for the cross-β motif, or does assembly result from interactions between amino acids in a disordered polypeptide chain? McKnight’s lab previously reported hallmarks of β structures in hydrogels and droplets formed from LC domains (e.g. Xiang et al., 2015; Kato et al., 2012; Oct 2016 news), but direct NMR of droplets revealed no such structures (Oct 2015 news).
Julie Forman-Kay, Hospital for Sick Children in Toronto, thinks the answer may not be one or the other, but all of the above. “People typically assume that β structure always leads to rigid and irreversible fibers; this paper shows it isn’t always the case,” said Forman-Kay. “They’ve shown a cross-β structured core, but it’s not an indestructible rock, it’s dynamic and regulated by post-translational modification.” That is consistent with the type of transient multivalent interactions that can drive liquid phase separations, she said.
Fawzi told Alzforum he will be curious to know the extent of β structure in the liquid forms, a question no one has definitely settled yet. In his NMR analysis of FUS-LC droplets, the domain appeared highly mobile, with disorder spread across the entire sequence. But, he said, his techniques may not detect transient β structure, and will not observe stable polymers including the amyloid presented here.
The present structure will help resolve the debate, said Alberti. Now investigators can design specific mutations to disrupt the structure, and test their effects on phase separation, hydrogel formation, an ultimately FUS aggregation and toxicity in cells. “This can tell us if the structure is something physiological or only happens in a pathogenic state,” said Alberti.
One limitation of the study is its focus on the isolated LC domain, rather than full-length FUS protein, said Dorothee Dormann, Ludwig-Maximilians-Universität, Munich. Several studies support the idea that domains and residues outside the low-complexity domain contribute to phase transition of full-length FUS protein, she said. “I’m not sure that the cross-β structures observed here are the only key—one should see if the findings hold up in full- length protein,” Dormann told Alzforum.
Fawzi said he would also like to know how pathogenic ALS-associated mutations affect the structure.
McKnight declined to speak with Alzforum for this story.—Pat McCaffrey
References
News Citations
- Phosphorylation of FUS Does Away with Droplets
- ALS Research ‘Gels’ as Studies Tie Disparate Genetic Factors Together
- Do Membraneless Organelles Host Fibril Nucleation?
Paper Citations
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012 May 11;149(4):768-79. PubMed.
- Xiang S, Kato M, Wu LC, Lin Y, Ding M, Zhang Y, Yu Y, McKnight SL. The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei. Cell. 2015 Nov 5;163(4):829-39. PubMed.
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012 May 11;149(4):753-67. PubMed.
Further Reading
Primary Papers
- Murray DT, Kato M, Lin Y, Thurber KR, Hung I, McKnight SL, Tycko R. Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell. 2017 Sep 16; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Hospital for Sick Children/ University of Toronto
This paper describes an elegant solid-state NMR structural characterization of a cross-β conformation within an ordered 57-residue element of the FUS low-complexity (LC) region. It provides an important piece to the puzzle of what interactions drive protein phase separation, an area that is currently being studied intensely due to its critical role in biological compartmentalization and biomaterial formation.
The conformational landscape of the FUS-LC region is complex. Previous solution NMR studies, as well as data from this current work, demonstrate dynamic disorder, but pelleting fibrils for study by solid-state NMR enables signal to emerge from the equilibrium sampling of more ordered conformers. While it has previously been shown that the FUS-LC in a liquid phase-separated state can remain disordered, avoiding significant population of β structure, this data demonstrating cross-β structure that can assemble into fibrils is consistent with the observation of FUS fibers in hydrogels.
It is not clear yet how these different conformational states fit into the physiological function of FUS or the pathogenesis in ALS and FTLD, but it is obvious that the physical chemistry enables such β structures to be sampled. Transient β interactions could certainly contribute to the mix of multivalent interactions needed to drive liquid phase separation, along with pi-pi, cation-pi, electrostatic, and hydrophobic interactions that have been discussed in the literature.
That this structure is significantly less stable than many cross- β fibril states, enabling their dissolution by phosphorylation, argues for the role of such transient interactions within a physiologically relevant and dynamically regulated equilibrium.
Brown University
This is an important study that will stimulate a lot of additional work in the field. The observation that the structure is distinct from disease amyloid fibrils is of high interest for understanding assembly of not only FUS but many other low-complexity and "prion-like" domains. The existence of ALS-associated mutations (e.g. S57del, S96del) that map to the core region of the fibril structure presented by Murray et al. brings up the question if the mutations inappropriately over-stabilize this structure (or a related structure) leading to aggregation observed previously (Murakami et al., 2015).
Regarding the structure in the liquid forms, in our previous work (Burke et al., 2015) we examined a liquid-liquid phase separated (LLPS or "droplet") state formed by residues 1-163 of FUS via solution NMR. There we saw predominant disorder across the domain and very intense signals, suggesting we were observing the majority of the conformations. However, 1) those solution NMR techniques are not sensitive to small populations, and 2) large, rigid structures such as polymeric β-sheet assemblies would be invisible to our techniques. Furthermore, our results did not identify the molecular details of the contacts that mediate the liquid form. The molecular details of phase separation can indeed involve secondary structure—for example, in collaboration with Jeetain Mittal (Lehigh) we recently suggested that α-helical packing contributes to phase separation of TDP-43 (Conicella et al., 2016).
Going forward, it will be exciting to directly probe these contacts in LLPS states and to measure the amount of β-sheet (and α-helix) structure in the liquid forms both in vitro and in vivo, not only of FUS but of other phase-separated assemblies. Recent solution NMR work by Simon Sharpe as well as Julie Forman-Kay and Lewis Kay on phase separation is starting to directly probe the interactions that stabilize phase separation of other sequences (Reichheld et al., 2017; Brady et al.; 2017).
The observation that phosphosites that map to the core fibril region have the biggest contribution to droplet formation is exciting. The effect of phosphorylation in general is highly complementary to our recent study (in collaboration with Frank Shewmaker, USUHS) showing that phosphorylation and mimicking phosphorylation with charged amino acid substitutions can disrupt phase separation, aggregation, and cellular toxicity of FUS (Monahan et al., 2017), which was inspired by the seminal work from McKnight and coworkers on the effect of phosphorylation on FUS assembly (Han et al., 2012; Kato et al., 2012; Kwon et al., 2013).
In fact, I first heard about FUS when Rob Tycko invited Steven McKnight to speak about his work on FUS in 2012, before these papers were published. It was right before I started my own lab and was such a great area that he opened up that I decided to write my first independent grant applications on this topic. Steven McKnight was kind enough to support my applications and he and Masato Kato provided the initial plasmids.
References:
Murakami T, Qamar S, Lin JQ, Schierle GS, Rees E, Miyashita A, Costa AR, Dodd RB, Chan FT, Michel CH, Kronenberg-Versteeg D, Li Y, Yang SP, Wakutani Y, Meadows W, Ferry RR, Dong L, Tartaglia GG, Favrin G, Lin WL, Dickson DW, Zhen M, Ron D, Schmitt-Ulms G, Fraser PE, Shneider NA, Holt C, Vendruscolo M, Kaminski CF, St George-Hyslop P. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015 Nov 18;88(4):678-90. Epub 2015 Oct 29 PubMed.
Burke KA, Janke AM, Rhine CL, Fawzi NL. Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol Cell. 2015 Oct 15;60(2):231-41. Epub 2015 Oct 8 PubMed.
Conicella AE, Zerze GH, Mittal J, Fawzi NL. ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure. 2016 Sep 6;24(9):1537-49. Epub 2016 Aug 18 PubMed.
Reichheld SE, Muiznieks LD, Keeley FW, Sharpe S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proc Natl Acad Sci U S A. 2017 May 30;114(22):E4408-E4415. Epub 2017 May 15 PubMed.
Brady JP, Farber PJ, Sekhar A, Lin YH, Huang R, Bah A, Nott TJ, Chan HS, Baldwin AJ, Forman-Kay JD, Kay LE. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc Natl Acad Sci U S A. 2017 Sep 11; PubMed.
Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O'Meally R, Dignon GL, Conicella AE, Zheng W, Best RB, Cole RN, Mittal J, Shewmaker F, Fawzi NL. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017 Oct 16;36(20):2951-2967. Epub 2017 Aug 8 PubMed.
Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012 May 11;149(4):768-79. PubMed.
Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012 May 11;149(4):753-67. PubMed.
Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, McKnight SL. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell. 2013 Nov 21;155(5):1049-1060. PubMed.
Make a Comment
To make a comment you must login or register.