In PD Model, α-Synuclein Spreads from Intestine to Brain
Quick Links
The hypothesis that Parkinson’s disease could arise within the belly, not the brain, now gains support with a new mouse model. Valina Dawson of Johns Hopkins University, Baltimore, reported at a joint symposium held June 16-21 in Keystone, Colorado, that α-synuclein fibrils injected into the gut seeded the misfolding of normal mouse α-synuclein there. Over the course of 10 months, the aggregates spread into the midbrain and ultimately the cortex, where they killed neurons and triggered motor and cognitive deficits. Snipping the vagus nerve protected the brain. The study, co-led by Hopkins’ Ted Dawson and Han Seok Ko, was published in Neuron on June 17.
The gut hypothesis of Parkinson’s disease emerged in 2003 when neuroanatomists Heiko Braak and Kelly Del Tredici, both at the University of Ulm in Germany, spotted α-synuclein-laden inclusions within the enteric nervous system of people who had died with PD (Braak et al., 2003, and Jul 2011 news series). They proposed a staging scheme in which α-synuclein pathology spread from the gut to the vagus nerve then into the brain stem, midbrain, and ultimately to higher brain regions. However, the intestinal origins of PD have been difficult to prove. Dawson told the audience at Keystone that people who died with Lewy bodies that had not yet spread beyond the vagus nerve would not have been diagnosed with PD during life.
At Keystone, Dawson described how the new mouse model proves that the intestinal origin is plausible—at least in mice. Co-first authors Sangjune Kim and Seung-Hwan Kwon injected preformed fibrils (PFFs) of mouse α-synuclein into muscle layers of the duodenum and pylorus, which connects this first part of the small intestine to the stomach. These regions are heavily innervated by the vagus nerve. One month later, the researchers detected mouse pSer129-α-synuclein (p-syn) within the pylorus and duodenum, as well as in the medulla oblongata of the brain, where the vagus nerve cell bodies reside. By three months, p-syn had cropped up in the amygdala, and had started to accumulate in the substantia nigra, hypothalamus, and prefrontal cortex. By seven months, traces of the pathology had spread to the hippocampus, striatum, and olfactory bulb. By 10 months, p-syn aggregates filled the hippocampus, prefrontal cortex, olfactory bulb, and striatum, but had diminished in the amygdala, ventral midbrain, and medulla oblongata (see image below).

Propagation from the Gut. One, three, seven, and 10 months after PFFs were injected into the gut, p-syn aggregates (red dots) turned up in the dorsal motor nucleus of the vagus nerve. (DMV). They also were identified in the locus coeruleus, substantia nigra pars compacta, substantia nigra reticulate, basolateral amygdala, hippocampus, hypothalamus, cortex, prefrontal cortex, and striatum. MO is the medulla oblongata and OB is the olfactory bulb. [Courtesy of Kim et al., Neuron, 2019.]
This pathological cascade did not occur when fibrils were injected into α-synuclein knockout animals, or if α-synuclein monomers or PFFs of human α-synuclein were used. Together, these data suggested that the injected mouse α-synuclein PFFs seeded the misfolding of endogenous mouse α-synuclein in the gut, which propagated in a retrograde fashion into and throughout the brain. Snipping the vagus nerve shortly after the PFF injection—effectively cutting off the access route of the pathology into the brain—also nipped the pathology in the bud (see image below).
How did this spread affect the brain? The number of dopaminergic neurons in the substantia nigra took a nosedive by seven months after the injection, and by 10 months, more than half of those neurons had died. Dopamine levels in the brain started to drop three months after injection, and continued to plummet thereafter. Compared with control mice, the PFF-injected animals took longer to climb down a pole, fell more quickly off a spinning rod, and had weaker grip strength seven months after injection. They also dawdled over nest building, poorly remembered the location of a hidden platform, and failed to distinguish between novel and familiar objects. Again, α-synuclein knockout mice injected with PFFs had no neurodegeneration or behavioral deficits.
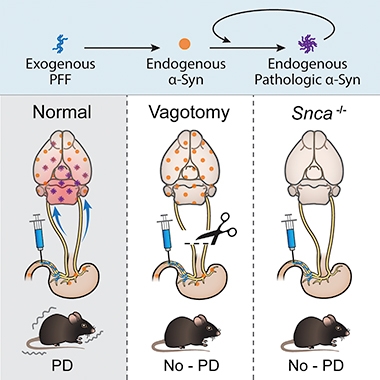
PD from the Gut? Injection of α-syn PFFs into the gut triggered the propagation of α-synuclein pathology into the brain (left), where it caused neurodegeneration. Vagotomy (middle) prevented the propagation and α-synuclein knockout mice were unaffected. [Courtesy of Kim et al., Neuron, 2019.]
Dawson pointed out that several other labs have tried, and failed, to spark PD-like pathology in the brain by targeting the mouse gut. She attributed her lab’s success to a number of factors, including their use of small fibrils, the hefty dose they injected (25 ug), their highly targeted injection adjacent to the vagus nerve, and how long they were willing to wait for the disease to unfold.
Aaron Gitler of Stanford University said that if it can be reproduced, the model could provide a useful way to study α-synuclein propagation from the gut. He said that the dependence on endogenous mouse α-synuclein suggests that the model is meaningful. Subhojit Roy of the University of Wisconsin in Madison agreed that the phenomenon was remarkable, but added that Dawson’s model still falls prey to the same artificiality that dogs all PFF injection models. “You do not see extracellular α-synuclein floating around in people with PD, so it is difficult to know how models like this relate to disease,” he said. “However the fact that extracellular α-synuclein can induce rapid intracellular aggregation is probably telling us something important.” Don Cleveland of the University of California, San Diego, wondered whether the pathological spread would be mitigated in mice expressing only a single copy of α-synuclein. Dawson has yet to test this.
In people, what would set off α-synuclein misfolding in the gut in the first place? Dawson suggested that problems with the gut microflora could somehow spark the process. There is evidence of an altered microbiome and inflammatory responses within the guts of PD patients (Dec 2016 news and May 2019 conference news).
Dawson also noted that levels of Poly ADP-ribose (PAR) are high in the gut. She previously implicated this branched chain ribose in aggregating and propagating α-synuclein in the brain (Kam et al., 2018).—Jessica Shugart
References
Series Citations
News Citations
- Do Microbes in the Gut Trigger Parkinson’s Disease?
- Do Immune Cells Promote the Spread of α-Synuclein Pathology?
Paper Citations
- Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003 May;110(5):517-36. PubMed.
- Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, Andrabi SA, Qi C, Poirier GG, Pletnikova O, Troncoso JC, Bekris LM, Leverenz JB, Pantelyat A, Ko HS, Rosenthal LS, Dawson TM, Dawson VL. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson's disease. Science. 2018 Nov 2;362(6414) PubMed.
Further Reading
Primary Papers
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson's Disease. Neuron. 2019 Aug 21;103(4):627-641.e7. Epub 2019 Jun 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.