MTBR-243 Tau: A Fluid Biomarker for Tangles Themselves?
Quick Links
Of tau’s 100 post-translational modification and truncation sites, phosphorylated tau-181 and tau-217 have received the most attention of late. These two species of this manifold protein appear in blood and CSF well over a decade before a person has symptoms. A paper published December 7 in Brain examines a different segment further down the 441 amino-acid-long tau protein, i.e., its microtubule-binding region. MTBR for short, it may become a biomarker to track progression closer to the symptomatic phase of this long disease—and maybe even lead scientists on the trail of the elusive tau aggregation “seed. “
- New extraction and mass spec techniques detect previously invisible parts of tau in CSF.
- MTBR-tau species beginning at residue 243 correlate tightly with tau PET and cognitive measures, flag tangles in AD.
- Antibodies targeting tau’s mid-region may be more effective.
Led by Kanta Horie and Randall Bateman at Washington University School of Medicine, St. Louis, the research team analyzed MTBR-tau species in AD and control CSF. They found that one fragment in particular, from residue 243 to 254, reflected changes in tau pathology that occur in AD.
What makes this fragment different than the better-known ones, which can be detected with simpler immunoassays? “There’s a current dogma in the field that phospho-tau species reflect tau tangles,” said Bateman. “I think the data is strong now that they absolutely do not. Phospho-tau species are increasing in response to amyloid plaques, not due to tau tangles.”
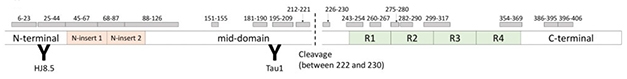
Cleaved tau. Researchers used chemical extraction, immunoprecipitation, and mass spec to analyze MTBR-tau species, which are located downstream from the cleavage site between residues 222 and 230. [Courtesy of Randall Bateman.]
MTBR-tau is the primary component of tau tangles. The ability to detect this piece of tau in CSF or, better yet, in blood, might enable scientists and physicians to infer when these aggregates have formed in a person’s brain. This could help confirm the stage of a person’s AD without an expensive PET scan, and to distinguish AD from other neurodegenerative diseases.
Scientists are unsure whether MTBR-tau is involved in extracellular tau aggregation. Previous mass-spectrometry studies have found that this tau region is enriched in aggregates in AD brains (Roberts et al., 2020). However, past tau biomarker studies didn’t find MTBR-tau in CSF, in part because the antibodies available at the time were insufficiently specific or sensitive. They instead focused on the parts of tau they could detect, that is, the N-terminal and mid-domain, aka proline-rich region, which are actively secreted from neurons after truncation (Sato et al., 2018).
To take a new crack at measuring the MTBR in body fluids, Bateman and colleagues worked out an extraction procedure and mass spectroscopy to analyze CSF tau species. First, they examined insoluble extracts from AD and control brains, and compared these to CSF tau profiles. They found that the MTBR species containing residues 299 to 317 and 354 to 369 were more concentrated in the extract from AD versus control. A third, more upstream region of MTBR, from residues 243 to 254, was also enriched threefold in AD versus control brains.
Could they find these same forms of tau in CSF? With the new method, the CSF concentrations of tau’s C-terminus were similar to the concentrations of tau species containing the N terminus and mid-domain. In control participants, CSF concentrations of MTBR-tau species were 0.4 to 3.7 ng/ml, and 6.5 and 5.1 ng/ml for non-MTBR C-terminal tau species, which include residues 386 to 395 and 396 to 406. The concentrations of C-terminal tau species were similar to those of the mid-domain tau species, suggesting that the C-terminal end of tau is also truncated in neuronal cells and secreted extracellularly, just like the N-terminus.
To probe if these secreted, MTBR-containing snippets are relevant to AD, the scientists examined CSF from a cross-sectional cohort of 30 amyloid-negative controls, 12 people with non-AD cognitive impairment, as well as 58 amyloid-positive participants, including 18 with preclinical, 28 with very mild, and 12 with mild to moderate Alzheimer’s dementia. All three MTBR-tau species—243, 299, and 354—were present in both AD and control CSF, but their levels were higher in the amyloid-positive groups. The authors also found that the MTBR-tau species better distinguished control from disease stages than did tau’s N-terminal to mid-domain species. Of the three, CSF MTBR-tau-243 was the only one whose level rose continually through the AD stages.

New marker. In this case-control cohort, people with more advanced AD dementia (purple) had more MTBR-tau-243 in their CSF than early stage (green) or cognitively normal people with brain amyloid (red), and much more than people whose cognitive impairment was not due to AD (orange) or controls (blue). [Courtesy of Randall Bateman.]
The rising levels of MTBR-tau-243 across AD stages suggested that it may predict disease progression. To test this idea, the authors investigated if a participant’s MTBR-tau species correlated with his or her scores on the CDR-SB and MMSE. Again, MTBR-tau-243 in the amyloid-positive group was highly correlated with both, indicating that this MTBR-tau species differentiates clinical stage and disease progression.
From the cross-sectional cohort, 28 participants have been followed for up to nine years, so the scientists were able to measure the longitudinal trajectory of MTBR-tau in their CSF. MTBR-tau-243, 299, and 354 concentrations significantly increased over time in the amyloid-positive, but not the amyloid-negative group.
Lastly, the scientists found that participants’ CSF MTBR-tau-243 concentration correlated with flortaucipir uptake in a PET scan; for MTBR-tau-299 and 354, this was less true.
“This is a well-designed study with discovery and validation experiments using human frozen brain tissues and cross-sectional and longitudinal experiments with CSF,” said Tsuneya Ikezu from Boston University School of Medicine. Ikezu noticed some weaknesses, including the small sample size in the longitudinal part of the study, in which most of the groups have but one or two patients. Also, assessing p-tau181 and p-tau217 alongside MTBR-tau to compare these biomarkers using the same cohort would have made this paper more compelling, Ikezu said. Bateman readily agrees work remains to be done to directly compare these markers. His group is also working to measure the MTBR tau species in blood.

Hand in Hand. MTBR-tau-243 levels in CSF correlate tightly with neurofibrillary tangle pathology as measured by flortaucipir PET. [Courtesy of Randall Bateman.]
Overall, Bateman sees these biomarkers working in tandem, with phospho-tau detecting the presence of amyloid plaques 15 to 20 years before symptom onset and MTBR-tau-243 pinpointing tangle deposition years later.
By way of analogy, Bateman quoted cardiovascular medicine. “A lipid panel for a patient isn’t just one marker — it’s HCL, LDL, cholesterol, and more,” Bateman said. “I think that’s where we’re heading with AD. We’ll have a panel of biomarkers that say something about tangle pathology, phosphorylated tau’s reaction to amyloid plaques, amyloid deposits, and general neurodegeneration. All of these pieces describe different dimensions of AD.”
The findings support the idea that antibodies targeting the mid-region of tau may be more effective at slowing disease spread than N-terminal ones (Apr 2018 news). Scientists are waiting to confirm this hypothesis, while mid-region targeting therapeutic antibodies such as JNJ-63733657, Bepranemab, and others are wending their way through clinical trials.—Helen Santoro
References
News Citations
Therapeutics Citations
Paper Citations
- Roberts M, Sevastou I, Imaizumi Y, Mistry K, Talma S, Dey M, Gartlon J, Ochiai H, Zhou Z, Akasofu S, Tokuhara N, Ogo M, Aoyama M, Aoyagi H, Strand K, Sajedi E, Agarwala KL, Spidel J, Albone E, Horie K, Staddon JM, de Silva R. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease. Acta Neuropathol Commun. 2020 Feb 4;8(1):13. PubMed.
- Sato C, Barthélemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, Kanaan NM, Yarasheski KE, Baker-Nigh A, Benzinger TL, Miller TM, Karch CM, Bateman RJ. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018 Mar 21;97(6):1284-1298.e7. PubMed.
Further Reading
Primary Papers
- Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021 Mar 3;144(2):515-527. PubMed. Correction.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.