A Minority of Human Aβ Species are Toxic, Good Drug Targets
Quick Links
Amyloid-β can assume a dizzying variety of shapes and sizes, and it is not clear which are most nefarious or even if they are found in the brain. Now, scientists led by Dominic Walsh at Brigham and Women’s Hospital in Boston report that only a tiny fraction of the oligomers in the human brain are toxic. As described in the April 23 Acta Neuropathologica, the researchers used a gentle extraction procedure that released all of the Aβ neurotoxicity yet only a small quantity of the soluble Aβ from human brain tissue. In a second paper in the July 11 Nature Communications, they detail how a human neuron-based assay for Aβ toxicity identified an oligomer-binding antibody, called 1C22, that protects neurons better than do anti-Aβ antibodies currently used in clinical trials. Dennis Selkoe, also from Brigham’s, was co-senior author on that paper. Together, the two papers suggest ways to home in on promising antibody targets for AD. “One reason why certain antibodies have not succeeded [in the clinic] might be that they get bound up on inactive targets,” said Walsh. “If you want real target engagement, you have to get after the active species of Aβ.”
- In the brain, toxic Aβ species are the minority.
- A gentle extraction method isolates them.
- A cell-based assay identifies the best antibodies to neutralize them.
“There are a plethora of different synthetic Aβ oligomers, and which, if any, is found in the AD brain has remained controversial,” wrote David Morgan, Michigan State University, Grand Rapids. "This work goes a long way towards identifying an antibody that appears to inhibit the activity of a dendritotoxic Aβ species from AD brain tissue.”
Walsh’s group discovered that by gently soaking tissue they could isolate Aβ species that freely diffuse, leaving behind other soluble species typically released by homogenization. Though this soaking extract had less Aβ and fewer oligomers than homogenates, it contained all of the toxicity. “Since these extracts contain the toxic species, we can now try to find agents that protect against them,” said Walsh.
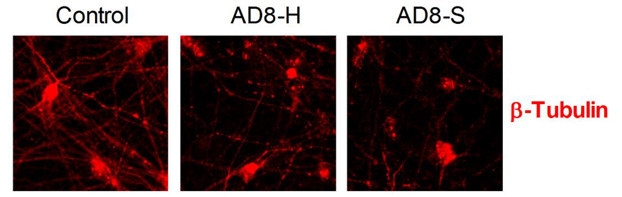
Potent Extracts. Soaking AD brain tissue released soluble forms of Aβ (AD8-S) that shriveled neurites much like brain homogenates (AD8-H). Homogenates from control brains had no effect. [Image courtesy Hong et al., 2018.]
Before this work, none of the anti-Aβ antibodies being used in clinical trials had been tested on human neurons treated with extracts from human brains, said Walsh. He believes that is a problem. Because most therapeutic anti-Aβ antibodies were raised to synthetic Aβ and tested in transgenic mice, it is unclear if they bind to the most toxic species in the human brain.
Ming Jin, co-first author on the Nature Communications paper with Brian O’Nuallain, developed an assay that uses all human material. To neurons derived from induced pluripotent stem cells from a healthy volunteer, Jin added Aβ-containing brain extracts prepared postmortem from one of five different AD patients. After three days, neurite lengths shrank by half, dendrites and axons lost branches, and the neurons began accumulating phosphorylated tau (p-tau).
When added at concentrations similar to those found in the brain during immunotherapy, anti-Aβ antibodies neutralized the extracts to different degrees. The murine precursors to solanezumab (mAβ 266), which binds best to Aβ monomers, and to bapineuzumab (mAβ 3D6), which binds both monomers and protofibrils, conserved neurite length and branching by about 60 and 75 percent, respectively.
The best protection came from 1C22. O’Nuallain generated this antibody using protofibrils. It weakly recognizes an epitope exposed in monomers and oligomers but buried in fibrils. However, being bivalent, it has stronger affinity for molecules with more than one epitope, such as oligomers and protofibrils. Over three days, 1C22 protected neurite length and branching from Aβ in AD brain extracts by about 85 percent (see image below). It also prevented the accumulation of p-tau better than did either m266 or 3D6.

Comparing Antibodies. 1C22 (dark blue) better preserved neurite length (left) and dendritic branching (right) than did 3D6 (cyan) and 266 (orange). A control antibody, 46-4, that targets a non-Aβ protein had no effect. [Courtesy of Jin et al., 2018.]
The results suggest that 1C22 should be used further as a research tool. Walsh has no plans to develop it as a therapeutic candidate. “1C22 is useful, but I expect it will be possible to make an antibody or find a small molecule that works better,” he said.
In collaboration with Sanofi in Bridgewater, New Jersey, Walsh and Selkoe have used the cell assay to test humanized monoclonal antibodies that have been used in clinical trials. Jin will present preliminary data at the Alzheimer’s Association International Conference in Chicago this month. He found that 1C22 protected neurons best, followed closely by SAR 228810, an antibody that binds Aβ protofibrils and fibrils and is currently in Phase 1, then by aducanumab and bapineuzumab. BAN2401 also protected neurons, but less well than the others. Aducanumab and BAN2401, which also binds protofibrils of Aβ, have shown some positive hints in clinical testing (May 2018 conference news; Jul 2018 news). “Collectively, these data suggest that better therapeutic antibodies can be found,” Walsh said. “All in all it is very encouraging.”
Donna Wilcock, University of Kentucky, Lexington, said that while the cell-based assay is useful, it can’t predict success in a clinical trial. “I think it adds another tool that we can use to characterize all these different antibodies,” she told Alzforum. However, even if an antibody didn’t neutralize the toxic Aβ in this assay, it might still work in vivo by engaging microglia or utilizing the peripheral sink mechanism, she said. On the other hand, even if an antibody did protect human-derived neurons against toxic Aβ, it might elicit too strong an immune response to be safe. “This assay won’t help inform the holistic approach in the brain.”
In Acta Neuropathologica, first author Wei Hong and colleagues describe how they used the same induced neuron-based assay to test their gentler soaking method for making brain extracts. In this case they took 0.5 mm-thick slices of previously frozen human tissue, soaked them in buffer for 30 minutes while shaking gently, then centrifuged to get a clear solution.
Compared with homogenization, the soaking method yielded less than a third the amount of Aβ42 monomers and oligomers for each gram of brain tissue. However, it more potently shrank neurites in induced human neurons and blocked long-term potentiation in hippocampal slices from mice. Further, homogenates of the tissue left behind after soaking had no effect on human neurons. “That suggests that the material released by homogenization is essentially inactive,” Walsh told Alzforum. The toxic species are instead hovering in the soluble fraction.
The data also suggest that oligomers come in both toxic and nontoxic forms, since all extracts contained some oligomers. A therapy that binds all oligomers might be less effective than one that targets just the toxic ones, Walsh said. His group is trying to isolate the toxic species in the extracts prepared by soaking tissue. “If we could understand what the bioactive species are, and figure out how to neutralize them, that could open up completely new routes of therapeutic intervention,” Walsh said.—Gwyneth Dickey Zakaib
References
Therapeutics Citations
Antibody Citations
News Citations
Further Reading
Papers
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012 Jan 29;15(3):349-57. PubMed.
- Viola KL, Klein WL. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015 Feb;129(2):183-206. Epub 2015 Jan 22 PubMed.
Primary Papers
- Hong W, Wang Z, Liu W, O'Malley TT, Jin M, Willem M, Haass C, Frosch MP, Walsh DM. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer's disease brain. Acta Neuropathol. 2018 Jul;136(1):19-40. Epub 2018 Apr 23 PubMed.
- Jin M, O'Nuallain B, Hong W, Boyd J, Lagomarsino VN, O'Malley TT, Liu W, Vanderburg CR, Frosch MP, Young-Pearse T, Selkoe DJ, Walsh DM. An in vitro paradigm to assess potential anti-Aβ antibodies for Alzheimer's disease. Nat Commun. 2018 Jul 11;9(1):2676. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Hertie Institute for Clinical Brain Research, University of Tuebingen
The paper by Hong et al. represents a great step toward improving the current technology used for Aβ extraction. The authors describe a gentle method that avoids harsh homogenization by soaking human brain slices in isotonic buffer. Although the authors restricted their extraction largely to diffusible Aβ species, this did not lead to loss of Aβ to the extent one may have expected, and it still allowed the authors to perform detailed characterization of the recovered Aβ species. Remarkably, removing soluble Aβ species by soaking brain slices in isotonic buffer completely eliminated neurotoxicity [in the tissue] in the assays performed. In addition, no further active Aβ was extracted upon subsequent homogenization, even though more Aβ was released than during soaking.
This data strongly suggests a link between Aβ toxicity and diffusible Aβ species. However, the relevance of the neurotoxicity assays used (neurite outgrowth, LTP) as well as all other in vitro Aβ toxicity assays for AD pathogenesis remains unclear and is debated. Interestingly, in size exclusion chromatography analysis, diffusible Aβ species recovered by this new method distribute widely from the low kDa into the 1,000 kDa range and show enrichment of lower molecular weight species compared to soluble Aβ extracted by homogenization. It seems likely that stronger differences occur in the even higher molecular weight range but low solubility of these species hinders analysis. Irrespective of these limitations, which are inherent to the field, this paper describes an advanced tool to enrich diffusible Aβ and provides an excellent basis for further detailed studies.
Acumen Pharmaceuticals
Prevail Therapeutics Inc.
The authors of these two papers from MGH should be congratulated for further characterizing which species of Aβ oligomers may be most toxic, and for developing methods to rapidly test various antibodies for protective properties in vitro. We also wish to point out a very recent publication (Wang et al., 2018) by Brian Bacskai and collaborators at MGH and Acumen Pharmaceuticals, which describes an in vitro assay using calcium dysregulation as a measure of neuronal toxicity. They characterized antibody ACU3B3, which selectively targets subspecies of soluble Aβ oligomers and abolishes their toxic effects in primary neuronal cultures. Based in part on this emerging technology, we agree that anti-Aβ antibodies specifically or at least preferentially targeting the bioactive aggregate species may lead to effective treatments for Alzheimer’s disease.
Eric Siemers is consulting CMO and Franz Hefti is a board member of Acumen Pharmaceuticals, Inc., a company developing ACU193, the humanized form of antibody ACU3B3.
References:
Wang X, Kastanenka KV, Arbel-Ornath M, Commins C, Kuzuya A, Lariviere AJ, Krafft GA, Hefti F, Jerecic J, Bacskai BJ. An acute functional screen identifies an effective antibody targeting amyloid-β oligomers based on calcium imaging. Sci Rep. 2018 Mar 15;8(1):4634. PubMed.
University of Minnesota
The article by Wong and colleagues is comprehensive and the experiments are rigorous and well-designed. The report’s main conclusion is that the pool of bioactive Aβ oligomers constitutes a minority of soluble Aβ in AD brain tissue. The results also suggest that not all pools of Aβ assemblies are neurotoxic.
In practical terms, these findings further support the notion that not all Aβ oligomers are equal in altering neuronal biology and function, a point our own studies have demonstrated when comparing Aβ dimers, trimers, Aβ*56, and 150kDa Aβ protofibrils purified from AD or APP transgenic mouse brains (Amar et al., 2017; Larson et al., 2012; Lesné et al., 2006; Sherman et al., 2016). The low abundance of the critical bioactive Aβ species present in the AD brain lysates is also consistent with the estimated rarity of soluble Aβ dimers, trimers, or Aβ*56 in the extracellular-enriched brain lysates generated by our group or in human cerebrospinal fluid or conditioned medium of APP transgenic neurons (Amar et al., 2017; Larson et al., 2012; Lesné et al., 2006, 2013; Sherman et al., 2016). This is particularly obvious when comparing Aβ pools present in extracellular-enriched fractions and in membrane-enriched fractions (Lesné et al., 2013; Sherman and Lesné, 2011). Further supporting these observations, the amount of each Aβ oligomer purified from brain tissue in our own studies varies between 0.5-5 ng/mg of extracellular-enriched total protein lysate (Amar et al., 2017).
The other major take-home message from this report is the necessity to assess the biological properties of distinct Aβ oligomers, to identify the exact Aβ assembly causing these functional alterations and to move away from synthetic Aβ mixtures. This point has crucial therapeutic repercussions because the target specificity for distinct Aβ oligomers of all Aβ antibodies recently used in clinical trials of AD (i.e., solaneuzimab, bapineuzimab, aducanumab) is unknown. Until this central information is available, it will remain challenging to fully interpret the reasons behind a failure or a success in these trials.
References:
Amar F, Sherman MA, Rush T, Larson M, Boyle G, Chang L, Götz J, Buisson A, Lesné SE. The amyloid-β oligomer Aβ*56 induces specific alterations in neuronal signaling that lead to tau phosphorylation and aggregation. Sci Signal. 2017 May 9;10(478) PubMed. Expression of Concern.
Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, Aguzzi A, Lesné SE. The complex PrP(c)-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer's disease. J Neurosci. 2012 Nov 21;32(47):16857-71a. PubMed.
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006 Mar 16;440(7082):352-7. PubMed. RETRACTED
Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013 May;136(Pt 5):1383-98. PubMed. Correction.
Sherman MA, Lesné SE. Detecting aβ*56 oligomers in brain tissues. Methods Mol Biol. 2011;670:45-56. PubMed.
Sherman MA, LaCroix M, Amar F, Larson ME, Forster C, Aguzzi A, Bennett DA, Ramsden M, Lesné SE. Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport. J Neurosci. 2016 Sep 14;36(37):9647-58. PubMed.
Make a Comment
To make a comment you must login or register.