LipiDiDiet Data Published
Quick Links
A paper published online October 30 in The Lancet Neurology details the outcome of the two-year LipiDiDiet trial. Researchers tested Souvenaid, made by Nutricia of Danone Research, in people with prodromal Alzheimer’s disease. Alzforum reported the preliminary results presented at the Athens/Springfield Symposium on Advances in Alzheimer Therapy last year (Mar 2016 conference news). The trial missed its primary cognitive endpoint, but met two of the secondary endpoints, suggesting the drink may have benefitted function and hippocampal atrophy.
- In the LipiDiDiet trial, Souvenaid missed its primary endpoint.
- It met two secondary endpoints, slowing hippocampal atrophy and functional decline.
- Authors debate which cognitive tests to use in prodromal disease.
The active ingredient in the daily breakfast drink is a formulation called Fortasyn Connect, which comprises antioxidants, essential fatty acids, and other molecules that are claimed to promote synaptic health. Tested for two years in 311 participants, this supplement failed to slow decline relative to controls on the primary cognitive outcome measure—the neuropsychological test battery (NTB)—in the cohort as a whole, what trialists call the modified intention-to-treat group (mITT).
Neither did the drink slow decline on the NTB memory domain. However, when the researchers analyzed the subgroup who stuck most closely to the daily regimen—the roughly 87 percent who were considered “per protocol,” or PP—the drink did temper the decline on both NTB scores. Since the mITT better reflects real life, where a patient may sometimes forget to take his or her daily supplement, it is more informative when deciding whether a drug works, said Pieter Jelle Visser, VU University Medical Center, the Netherlands, a co-author on the study. The PP analysis is more informative from a mechanistic perspective, he added.
Souvenaid curbed hippocampal atrophy and delayed decline on the clinical dementia rating-sum of boxes (CDR-SB) score (see image below). The latter was presented as a trend at the Athens conference, but errors have since been identified and scores corrected for baseline MMSE.
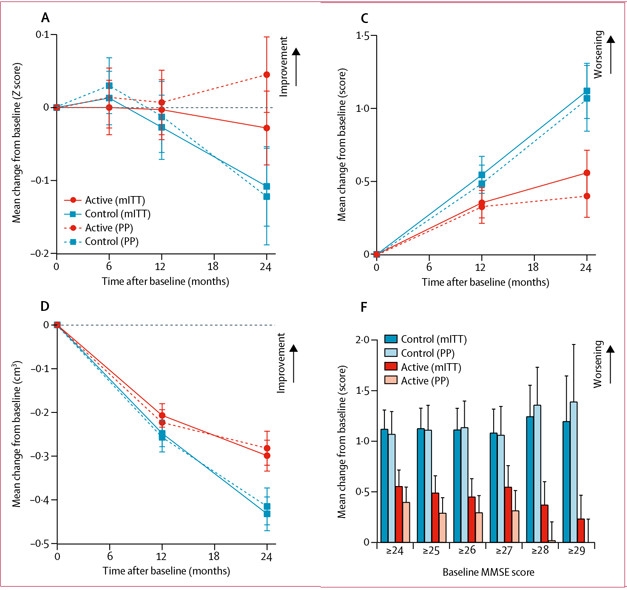
Mixed results: Souvenaid did not improve NTB scores (A) relative to the control beverage, except in the most compliant subgroup. The drink did delay decline on the CDR-SB (C) and atrophy of the hippocampus (D). Treated individuals with highest initial MMSE scores slipped the least on the CDR-SB (F). [©2017 American Medical Association. All rights reserved.]
Visser said he thinks there is a benefit to Souvenaid in prodromal AD, but more studies are needed to define it. In hindsight, the authors wonder if the NTB was the best choice for primary endpoint in this prodromal population. They initially designed this prodromal study, which began recruiting in 2009, based on the 12-month decline on the NTB observed in patients with mild to moderate AD (Harrison et al., 2007). However, controls fell by only a quarter of that amount, limiting the study’s power. By contrast, CDR-SB scores worsened in the control group by 12 months as expected. After this study was underway, U.S. and EU regulatory agencies advised using the CDR-SB to test for drug effects in prodromal AD (FDA Guidance; EMA Guidance).
Other researchers, including Jason Hassenstab, Washington University in St. Louis, noted the CDR-SB is not perfect, either. “The CDR-SB has its own challenges, with strong ceiling effects in prodromal populations and the possibility of detecting functional decline not always related to neurodegenerative disease,” said Hassenstab, who was not involved in the LipiDiDiet trial. “If people are on the cusp of normal, you run into problems using CDR-SB as an endpoint.” He agreed that there may be a benefit to Souvenaid, but reiterated that the study was underpowered to detect it. Researchers need better cognitive tests, with fewer practice effects and more frequent sampling, for this population, he argued. The Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite (ADCS-PACC), although proposed for preclinical AD, may be useful in prodromal populations as well, Visser said (Jun 2014 news on Donohue et al., 2014).
Treated people with the highest baseline MMSE scores deteriorated the least on CDR-SB, implying that if this dietary intervention has any benefit, it works best early in disease, suggest the authors.
Hussein Yassine, University of Southern California, Los Angeles, agreed, saying the intervention in this trial may have come too late. People with higher MMSE scores did seem to do better, he wrote in an accompanying editorial. “Together with past trials, the LipiDiDiet does not provide sufficient evidence for the use of Fortasyn Connect in mild or prodromal Alzheimer’s,” he wrote. “The suggestion of benefit in two of the secondary outcomes is encouraging, but needs to be confirmed in further research.”
Visser said the outcome may also have been influenced by the heterogeneity of the participants, because the definition of prodromal disease when LipiDiDiet started enrolling in 2009 included people who had either cerebrospinal fluid, positron emission tomography, or magnetic resonance imaging markers of the disease. This was the first large trial started after the first version of the IWG research criteria were published (Dubois et al., 2007). Given what has been learned since then about how to classify the pre-dementia stages of Alzheimer’s, Visser now recommends restricting enrollment to those with evidence of amyloid pathology in order to zero in on people with true prodromal AD.
Investigators will soon complete a LipiDiDiet extension trial, which followed a subset of subjects for up to four years. Another ongoing project will examine the effect of Souvenaid on glucose metabolism in the brain using FDG-PET. Patrick Kamphuis, who leads the neuroscience division of Nutricia Research, said there would be no change to the company’s marketing of Souvenaid as a result of the LipiDiDiet data.—Gwyneth Dickey Zakaib
References
Therapeutics Citations
News Citations
- Souvenaid Trial Missed Primary, Partially Met Secondary Endpoints
- Test Battery Picks Up Cognitive Decline in Normal Populations
Paper Citations
- Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007 Sep;64(9):1323-9. PubMed.
- Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, Weiner M, Aisen PS, Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, Alzheimer’s Disease Neuroimaging Initiative, Alzheimer’s Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014 Aug;71(8):961-70. PubMed.
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007 Aug;6(8):734-46. PubMed.
External Citations
Further Reading
News
- Aging Mice are Sharper, Fitter on a High-Fat, Low-Carb Diet
- New Dementia Trials to Test Lifestyle Interventions
- CTAD: EEG Gains Luster as More Trials Incorporate Biomarkers
- Nutrient Formulation Appears to Grease Memory Function
- Neither Exercise Nor Supplements Boost Cognition in Two Large Studies
- Healthy Lives, Healthy Minds: Is it Really True?
Primary Papers
- Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, Hartmann T, LipiDiDiet clinical study group. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017 Dec;16(12):965-975. Epub 2017 Oct 30 PubMed.
- Yassine HN. Targeting prodromal Alzheimer's disease: too late for prevention?. Lancet Neurol. 2017 Dec;16(12):946-947. Epub 2017 Oct 30 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.