Aging Mice are Sharper, Fitter on a High-Fat, Low-Carb Diet
Quick Links
Eating mostly greasy foods like butter, bacon, and mayonnaise may sound unhealthy and unappetizing, but two new studies revive previously touted benefits for physical and mental health of such a diet, at least in mice. Eric Verdin at the Buck Institute for Research on Aging in Novato, California, and colleagues report in the September 5 Cell Metabolism that male mice on a ketogenic diet were likelier to reach old age than controls, while retaining their youthful heart health and memory capacities. In the same issue, José Alberto López-Domínguez, also at the Buck, and Jon Ramsey of the University of California, Davis, and colleagues independently report the same mortality benefit for male mice on a ketogenic diet, as well as preserved memory, strength, coordination, and endurance. Both groups analyze the signaling pathways involved.
- A ketogenic diet lengthened average lifespan and preserved fitness and memory in old mice.
- PPARα signaling went up, mTOR down.
- The diet, or supplements that mimic it, could support brain metabolism in AD.
This work may inform future therapies for age-related diseases such as Alzheimer’s, some researchers claim. Ketogenic diets are extreme high-fat regimens that induce production of ketone bodies in the blood, shifting the body into a metabolic alternative to glycolysis.
“The most important aspect of these papers is that they are finally breaking into the mechanisms that underlie the advantages of ketosis,” said Haakon Nygaard at the University of British Columbia, Vancouver. This was possible because the researchers designed ketogenic regimens that prevent obesity, and used control diets that were also high in fat but not ketogenic.
People on a ketogenic diet (KD) get roughly 85 percent of their calories from fat, while eating less than 50 grams of carbs per day, equivalent to a cup of cooked white rice. The diet first became popular in the 1920s when it proved helpful for curbing epileptic seizures, and is still used today for patients, mostly children, who fail to respond to standard anticonvulsant medication. More recent studies have hinted at broader benefits, including promoting weight loss, reducing hunger, and even countering age-related disease (Paoli et al., 2013). As a result, many people are giving KD a try, and dozens of products, ranging from dietary supplements to cookbooks, have sprung up, some claiming to increase energy, mental focus, fat burn, and athletic performance. Some are being marketed without robust efficacy data. For example, the nutritional beverage Ketasyn/AC1202, which contains ketone body precursors, missed its primary endpoint in a Phase 2 trial for AD, but was nevertheless sold as a medical food (Oct 2009 news).
The KD’s long-term effects and underlying mechanisms remain obscure. Indeed, at the outset of the study, Ramsey feared long-term KD might impair liver and kidney function.
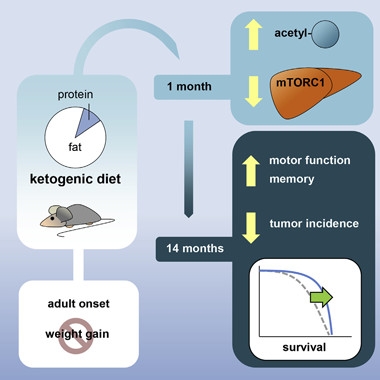
Fatty Foods to the Rescue?
A ketogenic diet helped mice survive to old age, and made them more physically and mentally fit. As in previous longevity studies, protein acetylation and PPARα activation rose, while mTOR signaling fell. [Courtesy of Roberts et al., Cell Metabolism.]
To test the effects of a KD on lifespan, first author Megan Roberts in Ramsey’s lab fed 12-month-old mice regular chow, a high-fat/low carb diet (HFD) that delivered 70 percent of calories as fat, or a ketogenic diet (KD) consisting of 89 percent fat. Only the latter boosted production of ketone bodies in the animals. Comprising acetoacetate, β-hydroxybutyrate (BHB), and acetone, ketone bodies are produced when the liver, starved of glucose, breaks down lipids to release fatty acids, which then undergo β-oxidation. Importantly, Roberts limited the amount of food so that all mice consumed the same number of calories and mice on the KD and HFD remained lean. She found that mice fed the KD lived, on average, 13.6 percent longer than mice on control chow. That is equivalent to seven to 10 human years, said Ramsey. Those on the HFD outlived controls by just a tad. Specifically, the KD enhanced a mouse’s chance of reaching old age without lengthening maximum lifespan.
Using a different regimen, first author John Newman at the Buck Institute obtained similar findings. Newman fed 12-month-old mice either control chow, a KD without any carbohydrates, or a high-fat non-KD. A separate group of mice alternated weekly between control and one of the higher-fat diets. As expected from a previous study (Douris et al., 2015), the mice eating high-fat diets ad libitum became obese, but mice on the cycling diets did not.
Surprisingly, the alternation also drove up BHB plasma levels. Verdin considers this a plus, because he hypothesizes that BHB is the main mediator of the KD’s effects. Newman observed that the cycling KD lowered a mouse’s risk of dying in middle age, while the cycling HFD was weakly beneficial.
In essence, when weight was kept under control by limiting calories or alternating between diets, mice on a KD were likelier to reach old age. This is consistent with the notion that caloric restriction, known to lengthen lifespan of several species, may be mediated through ketone bodies, which in turn are generated by calorie restriction or a KD (Veech et al., 2017).
“Developing diets that maintain high ketone levels without stimulating obesity is a challenge,” wrote Patrick Bradshaw at East Tennessee State University in Johnson City to Alzforum. Bradshaw found that BHB lengthens the life of Caenorhabditis elegans (Edwards et al., 2014). “The authors of both manuscripts insightfully accomplished this task, and identify that long-term ketogenic diets can extend median lifespan (healthspan) of mice,” he wrote.
Both groups reported that KD mice outperformed other mice on a range of health measures. In Roberts’ study, 26-month-old male mice who had eaten a KD for 14 months hung onto a wire longer, gripped a bar more tightly, moved faster, and reared up on their hind legs more often than mice on the control diet. Their hind limb muscles bulked up more. Mice on the high-fat, non-ketogenic diet were fitter than those on a control diet, but less so than KD mice. Though 22- to 24-month-old mice on the cycling KD did not beat control mice in tests measuring balance, activity, and strength, their cardiac health was better, matching that of three-month-old mice.
KD mice also did better on memory tests. Both teams found that mice eating the KD or cycling KD spent more time exploring new objects than did the other mice, and in Newman’s study, aged mice on the KD bested controls in a water maze test of spatial memory, or in remembering which section of a rotating platform would give them a foot shock.
“We were surprised by the number and the magnitude of the changes,” said Ramsey. “Mice on the KD showed no age-related decreases in memory, muscle strength, or endurance. This maintenance of youthfulness was probably the most impressive finding.”
Russell Swerdlow, University of Kansas, Kansas City, agreed. “The range of benefits shown is exciting,” he told Alzforum, noting consistency with some human studies. For example, ketone levels correlate with slight memory benefits in people with mild cognitive impairment (Krikorian et al., 2012), and caloric restriction, which induces ketogenesis, improved the memory of elderly adults (Witte et al., 2009).
Searching for associated molecular changes, Ramsey’s group focused on two signaling mechanisms previously connected to caloric restriction and longevity. After a month on the KD, lysine acetylation levels in the liver, which increase in response to reduced calories, shot up fivefold compared with mice on the other two diets. The finding fits with previous reports showing BHB can inhibit histone deacetylases (Shimazu et al., 2013). Acetylation of lysine 9 on histone 3 correlated with increased expression of FOXO3a, implicated in longevity, and MnSOD, implicated in stress-response pathways, even though mice on the high-fat, non-ketogenic diet also had these changes.
Ramsey and colleagues also examined the mTORC signaling pathway, known to wane under caloric restriction. Interestingly, while the KD dialed down mTORC1 signaling in the liver, it bolstered it in muscle. These different needs of each tissue may dictate their differential responses to the diet, said Ramsey.
Verdin’s group chose an unbiased survey of gene expression to uncover molecular mechanisms. Newman sequenced RNA extracted from liver and kidney after a week on diet. Unsurprisingly, the transcriptional profiles of mice fed the KD had much in common with those of mice fed the HFD. Nearly half of the genes downregulated by the KD were also downregulated by the HFD, including genes that support glucose metabolism, insulin signaling, fatty-acid synthesis, and TOR activity. Interestingly, the drop in mTOR signaling persisted in 26-month-old mice that had been on a cyclic KD for over a year, even when tested during a week when they were eating a control diet.
Newman also found a murine gene-expression pattern unique to the KD. It upregulated genes involved in fatty acid oxidation, genes that encode mitochondrial proteins, and targets of PPARα, a transcription factor that regulates lipid metabolism and switches on during energy deprivation to trigger ketone body production, or ketogenesis. A recent study proposed that PPARα acts as a calorie restriction mimetic (Barger et al., 2017). As in the case of the mTOR signaling downregulation, the upregulation of fatty acid oxidation genes and the PPARα targets persisted in 26-month-old mice, even on a control week of their cycling KD.
”The transcriptome data are the most valuable,” said Nygaard. Although some of the findings were unsurprising given that the epilepsy literature has already linked ketosis to mTOR and PPARα, Nygaard welcomed the systematic survey. “That they found this in a completely unbiased search, and it’s consistent with previous findings in epilepsy, is very exciting,” he said. Bradshaw agreed. He was particularly intrigued by one gene highly upregulated by the KD, the PPARa target PEPCK-c. “Overexpression of PEPCK-c in muscle has been shown to increase longevity, hyperactivity, appetite, and exercise endurance in mice,” he wrote.
What do the results mean for humans? “That diet is affecting so many aging processes that exist in humans gives us some level of confidence that the results may be meaningful for humans,” said Ramsey. He also acknowledged important differences: mice die more often from cancer and suffer less from cardiovascular disease than humans, for example. Indeed, Bradshaw suspects that cancer prevention explains a chunk of the KD’s lifespan extension. Newman reported that only the KD activated tumor suppressor proteins p53 and p21. The sensitivity of mice and humans to the KD may also differ. BHB plasma levels were much lower in KD mice than in epileptic patients on this diet, noted Nygaard, even though the mice were on a harsher, zero-carb diet. The exclusive use of male mice in the studies limits predictions of how a KD might affect women, especially since estrogen affects metabolism in the body and brain (Rettberg et al., 2013).
Are the results relevant to neurodegenerative disease, in particular Alzheimer’s? Some researchers think so, since brain glucose uptake and metabolism drop in AD, and ketone bodies are the main backup fuel for the brain (Cunnane et al., 2016). Indeed, Robert Pawlosky of the National Institute on Alcohol Abuse and Alcoholism in Bethesda, Maryland, recently showed that a ketogenic supplement increased intermediates of both the tricarboxylic acid cycle and glycolysis in the hippocampus of the triple transgenic 3xTg mouse model of AD (Pawlosky et al., 2017). Pawlosky also found elevated hippocampal n-acetyl aspartate, which correlated with decreased anxiety, a finding consistent with observations in humans. “This is where the field is headed,” said Verdin, who wants to see more studies examining the effects of ketone bodies in specific brain regions.
The ability of ketone bodies to calm the spiking of neurons in epileptic brains may also prove beneficial for AD, some researchers believe. People with AD have a higher incidence of epileptic activity, and this appears to correlate with accelerated cognitive decline (July 2013 news; Vossel et al., 2016). In a manuscript posted on the BioRχiv preprint server, Verdin and colleagues report that a KD dramatically reduced epileptiform spikes in transgenic J20 mice. The KD also improved the male mice’s memories and greatly decreased male mice's risk of dying from seizures through midlife. “The effect on spikes and memory are probably through both anticonvulsant effects and improved brain bioenergetics,” hypothesized Nygaard. “The idea that diet might help is exciting.”
If these potential benefits for neurodegenerative disease were confirmed, how to bring them to people? A KD is not haute cuisine. “The KD is oily, not very palatable, and can cause diarrhea,” said Bradshaw, A cyclic KD might be more tolerable, but Verdin cautioned that switching your metabolism weekly, and the accompanying weight swings, could be unhealthy, especially in older people. Indeed, he wondered whether this was the reason his group saw no increase in maximal lifespan.
Swerdlow reported in July at the Alzheimer’s Association International Conference (AAIC) in London that 10 patients with mild or very mild AD were able to stick to a three-month KD supplemented with medium chain triglycerides (MCT), ketone body precursors, without serious side-effects. Five patients with more advanced dementia failed to sustain ketosis levels and dropped out of the study. Swerdlow is now organizing a larger study to examine cognitive benefits and track insulin, ketones, inflammation and mitochondrial markers. He sees this as proof of principle to show brain metabolism can be modified, but envisions more targeted interventions in the future.
Focusing on MCTs only, Nygaard described pilot trials in AD and frontotemporal dementia at AAIC. Although MCTs, the purported active ingredients in Ketasyn/AC1202, have yielded disappointing results in AD clinical studies so far, Nygaard thinks the studies, lacking robust markers to trace the MCTs’ effects, are far from conclusive. “We’re trying to take the guesswork out of the equation,” he said. To do so, he is assembling a battery of biomarkers, including tracers of metabolic activity, functional magnetic resonance imaging, arterial spin labeling to monitor brain blood flow, and electroencephalography, which should help assess whether the supplements are tweaking metabolism and brain function as expected. His group is starting to test five doses of a mixture of MCTs with backbones that are eight or 10 carbons long in patients with mild to moderate AD.
Verdin and Nygaard think specific molecules like these are the future, not diets. Verdin is developing hybrid MCT-BHB compounds which, when injected into mice, release BHB quickly, with the MCT portion providing a longer-term source of ketone bodies. In wild-type mice, he found that C6-BHB beat other hybrids at increasing BHB blood levels. In J20 mice, it reproduced the anti-epileptic effects observed with the KD. Verdin is also interested in testing fibrate drugs that activate PPARα and are currently used for treating people with high triglycerides in blood. “We’re just scratching the surface,” said Ramsey. “There’s a lot of potential for developments.”—Marina Chicurel
References
News Citations
- Medical Foods—Fallback Option for Elusive AD Drug Status?
- Epilepsy in Alzheimer’s Can Be Early and Subtle
Research Models Citations
Therapeutics Citations
Paper Citations
- Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013 Aug;67(8):789-96. Epub 2013 Jun 26 PubMed.
- Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, Locasale JW, Maratos-Flier E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim Biophys Acta. 2015 Oct;1852(10 Pt A):2056-65. Epub 2015 Jul 11 PubMed.
- Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017 May;69(5):305-314. Epub 2017 Apr 3 PubMed.
- Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY). 2014 Aug;6(8):621-44. PubMed.
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012 Feb;33(2):425.e19-27. Epub 2010 Dec 3 PubMed.
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1255-60. Epub 2009 Jan 26 PubMed.
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013 Jan 11;339(6116):211-4. Epub 2012 Dec 6 PubMed.
- Barger JL, Vann JM, Cray NL, Pugh TD, Mastaloudis A, Hester SN, Wood SM, Newton MA, Weindruch R, Prolla TA. Identification of tissue-specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics. Aging Cell. 2017 Aug;16(4):750-760. Epub 2017 May 26 PubMed.
- Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer's Disease. Front Mol Neurosci. 2016;9:53. Epub 2016 Jul 8 PubMed.
- Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, Veech RL. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J Neurochem. 2017 Apr;141(2):195-207. Epub 2017 Mar 15 PubMed.
- Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, Nagarajan SS. Incidence and impact of subclinical epileptiform activity in Alzheimer's disease. Ann Neurol. 2016 Dec;80(6):858-870. Epub 2016 Nov 7 PubMed.
Further Reading
Papers
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, Verdin E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017 Sep 5;26(3):547-557.e8. PubMed.
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995 May;9(8):651-8. PubMed.
Primary Papers
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017 Sep 5;26(3):539-546.e5. PubMed.
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, Verdin E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017 Sep 5;26(3):547-557.e8. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.