Alzheimer’s, Primary Age-Related Tauopathy Take Separate Paths
Quick Links
Some older people accumulate tau in their medial temporal lobes but have no amyloid plaques, a condition called primary age-related tauopathy (PART). Do they eventually develop Alzheimer’s disease?
- In primary age-related tauopathy, tangles accrue only in the medial temporal lobe.
- Amyloid plaques are absent, and no-shows over three years.
- Tau accumulates, cognition slips, more slowly than in AD.
- CSF phospho-tau181 rose similarly in PART and AD.
At least not over a span of three years, according to researchers led by Pablo Aguiar, Universidade de Santiago de Compostela, Spain, Michael Schöll, University of Gothenburg, Sweden, and Michel Grothe, Instituto de Biomedicina de Sevilla, Spain. In the August 14 JAMA Neurology, they reported that people with PART continued to amass tangles, but at a slower rate than people with AD. Their cognition marginally declined but no amyloid plaques appeared.
Curiously, cerebrospinal fluid phospho-tau181, a commonly used marker of amyloid-related tauopathy, rose almost as much in PART as in AD. What this means is unclear. “P-tau181 is a work in progress, and the biofluid biomarkers related to AD neuropathologic change are a moving target but a fascinating and all-important one,” wrote Peter Nelson, University of Kentucky, Lexington (comment below).
Still, people with PART and AD clearly declined in distinct ways. “The differing biomarker and cognitive trajectories of the [two] groups refute the idea that [PART] may represent an early stage of, or an alternative pathway to, AD,” wrote Susan Landau, University of California, Berkeley, and Elizabeth Mormino, Stanford University School of Medicine, in a JAMA Neurology editorial.
About a decade ago, Nelson and colleagues proposed that PART was a separate disease from AD (Nov 2014 news). However, because PART was diagnosed at autopsy, longitudinal data about how these people progress, and if they develop AD, was unknown.
To find out what happens during PART, co-first authors Alejandro Costoya-Sánchez at U Santiago de Compostela and Alexis Moscoso at U Gothenburg analyzed amyloid and tau PET, structural MRI, CSF Aβ42/40 ratios, p-tau181 levels, and cognitive test scores from 965 participants from three cohorts: the Alzheimer’s Disease Neuroimaging Initiative (ADNI), the Harvard Aging Brain Study (HABS), and the AVID-A05 study. Half were women, their average age 74, 93 percent were Caucasian.
Of all participants, 250 were PET amyloid-negative (A-) and had no tau in their medial temporal lobes (TMTL-), 451 were A+TMTL+, meaning they had AD, and 264 were A-TMTL+, which the authors used as an in vivo analog of PART. “It seems like the A-TMTL+ phenotype overlaps very broadly with PART,” Nelson noted.
At baseline, people with AD had widespread tangles throughout their temporal, parietal, and frontal lobes, whereas in PART, tangles were mostly in the MTL. Over three years, PART participants accumulated few tangles, adding an average of 0.02 SUVR per year, mainly in their temporal lobes. People with AD, on the other hand, racked up almost three times as many tangles annually throughout their cortices (see image below).
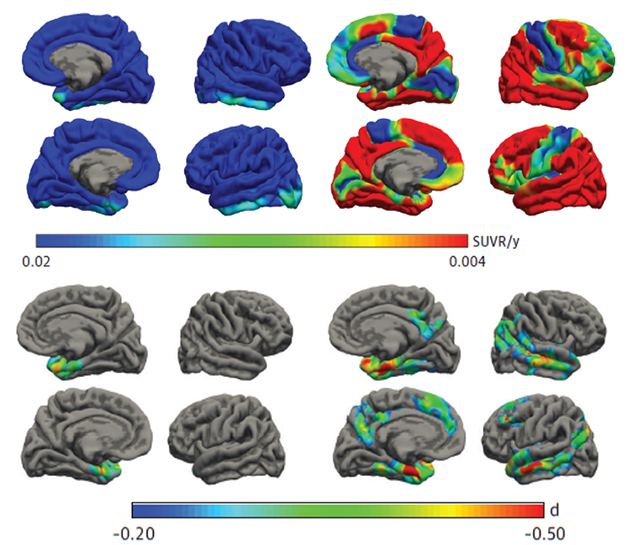
PARTial Tauopathy and Atrophy. In the PART brain, neurofibrillary tangles deposit only in the medial temporal lobe (top left), which shrank slowly over time (bottom left). In AD, tau accumulation (top right) and atrophy (bottom right) were widespread. [Courtesy of Costoya-Sánchez et al., JAMA Neurology, 2023.]
Atrophy and global cognitive decline mirrored tangle accumulation. In PART, only the MTL shrank, and people tallied an average of 1.6 points higher each year on the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog11), where a higher score means worse cognition. In AD, the entire temporoparietal region atrophied, and people added an average of 3.1 points annually to their ADAS-Cog11. “These results suggest that tau accumulation in amyloid-negative individuals is not a benign process, … although the rates of progression are significantly slower compared to [those] in amyloid-positive participants,” the authors wrote.
Amyloid accumulation diverged, as well. While AD participants added an average of three Centiloids per year, those with PART accrued no plaques over the short follow-up period of up to three years. This was true whether the scientists measured plaques via CSF Aβ42/40 or by PET using a low positivity threshold 12 Centiloids, rather than the more common 24.

P-Tau in PART. CSF p-tau181 levels start higher in AD (red) than in PART (green, left), but increase at similar annual rates (right). Controls in blue. [Courtesy of Costoya-Sánchez et al., JAMA Neurology, 2023.]
CSF p-tau181 bucked the trend. While it was lower in PART than AD at baseline, it rose at almost the same rate in both (see image above). What does it mean that a commonly used marker for AD-related tauopathy rose at a similar rate with or without amyloid? This p-tau isoform may not be exclusively related to amyloid-dependent tau pathology, but might reflect the type of tau tangles, say the researchers. “Tau deposits in both diseases consist of three-repeat/four-repeat neurofibrillary tangles,” Grothe wrote.
Henrik Zetterberg, also at U Got, interpreted the p-tau data differently. “Considering the magnitude of the absolute increase in CSF p-tau181 concentration in amyloid-positive compared with -negative individuals, we can conclude that amyloid pathology is the main driver of CSF p-tau181 increase, although a small, and likely clinically irrelevant, effect of non-amyloid-related tau pathology on CSF p-tau181 concentration cannot be excluded,” he wrote (comment below).—Chelsea Weidman Burke
References
News Citations
Further Reading
Primary Papers
- Costoya-Sánchez A, Moscoso A, Silva-Rodríguez J, Pontecorvo MJ, Devous MD Sr, Aguiar P, Schöll M, Grothe MJ, Alzheimer’s Disease Neuroimaging Initiative and the Harvard Aging Brain Study. Increased Medial Temporal Tau Positron Emission Tomography Uptake in the Absence of Amyloid-β Positivity. JAMA Neurol. 2023 Oct 1;80(10):1051-1061. PubMed.
- Landau SM, Mormino EC. Tau Pathology Without Aβ-A Limited PART of Clinical Progression. JAMA Neurol. 2023 Oct 1;80(10):1025-1027. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
The editorial by Landau and Mormino very nicely summarizes the key “takeaways” of this study by Costoya-Sanchez et al. The study recapitulates some conclusions based on clinicopathological work published by my colleague, Joseph L. Price, and me many years ago (Price and Morris, 1999), where we introduced the concept of preclinical Alzheimer’s disease. Our findings, in the brains of cognitively normal people aged 51-88 years at death, were that: 1) all individuals had neurofibrillary tangles (NFTs) in limbic structures and the density of these NFTs increased slowly with age; 2) this age-related tauopathy (now termed PART) does not represent a process that culminates in Alzheimer disease; and 3) the appearance of neocortical plaques is independent of this age-related tauopathy but has the effect of accelerating NFT formation such that it extends beyond medial temporal lobe structures.
It is reassuring that these new and impressive results from the deeply phenotyped participants in ADNI and the Harvard Aging Brain Study appear consistent with our findings of 25 years ago.
References:
Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999 Mar;45(3):358-68. PubMed.
Mayo Clinic College of Medicine
The amyloid-negative (A-)/flortaucipir (FTP) medial temporal lobe (MTL)-positive (FTP-MTL+) group has not previously received attention, and the current report helped to fill that gap nicely. This is a well-done and clearly written piece that makes the point that persons who are A-/FTP-MTL+ have the following consistent features:
1. They rarely accumulate amyloid at a rate seen in A+T+ persons. Note that the likelihood that most FTP retention in an A+T+ person is likely to be 3R/4R tau.
2. FTP accumulation is largely limited to the medial temporal lobe and does not spread into extra medial temporal regions.
3. There are slight reductions in medial temporal volume but not of the magnitude seen in A+T+ persons.
4. The increased FTP signal should not necessarily be assumed to represent 3R/4R tauopathy exclusively, but rather could be capturing 4R tauopathy or TDP43 proteinopathy.
My only criticism of the manuscript is a terminology one: I would have preferred that the authors refer to the TMTL+ as FTP-MTL+ to acknowledge the reality that it is the tracer that is “positive,” while there remains uncertainty whether the increased signal is truly 3R/4R tau, or off-target binding to something like TDP43. My bottom line is that A-/FTP-MTL+ is a non-Alzheimer’s neurodegenerative condition that has an indolent course.
University of Gothenburg
In most earlier studies, CSF p-tau181 concentration has not been associated with tau tangle pathology as determined by tau PET or neuropathological examination, although one study from the Swedish BioFINDER study found weak correlations between CSF p-tau181 concentration and tau PET signal in certain brain regions with Spearman correlation coefficients of around 0.10-0.15 (Wuestefeld et al., 2023). In a recent paper from our group (Erickson et al., 2023), we found that the prevalence of an A-T+ profile in CSF (where CSF Aβ42/40 ratio and p-tau181 were the biomarkers determining the A and T categories) was less than 5 percent. Longitudinally, no significant differences in rates of cognitive worsening were observed between A-T+ and A-T- profiles for cognition or imaging biomarkers. Cross-sectionally, A-T+ had similar tau PET signal to individuals with an A-T- biomarker profile.
The longitudinal increase of p-tau181 concentration in PART could be interpreted as an Aβ-related phenomenon, if p-tau181 was a more sensitive biomarker for amyloid pathology than amyloid PET. However, the negative CSF Aβ42/40 ratio in the PART group speaks against this interpretation (since CSF Aβ42/40 ratio is thought to be a very early amyloid marker). Nevertheless, it would have been interesting to examine what the data would have looked like if only CSF Aβ42 was used to classify people as amyloid-positive or -negative. Some data suggest that CSF Aβ42 concentration drops prior to the Aβ42/40 ratio when amyloid starts accumulating in the brain.
From all these data, considering the magnitude of the absolute increase in CSF p-tau181 concentration in amyloid-positive compared with -negative individuals, I think we can conclude that amyloid pathology is the main driver for CSF p-tau181 increase, although a small and clinically likely irrelevant effect of non-amyloid-related tau pathology on CSF P-tau181 concentration cannot be excluded.
References:
Wuestefeld A, Pichet Binette A, Berron D, Spotorno N, van Westen D, Stomrud E, Mattsson-Carlgren N, Strandberg O, Smith R, Palmqvist S, Glenn T, Moes S, Honer M, Arfanakis K, Barnes LL, Bennett DA, Schneider JA, Wisse LE, Hansson O. Age-related and amyloid-beta-independent tau deposition and its downstream effects. Brain. 2023 Aug 1;146(8):3192-3205. PubMed.
Erickson P, Simrén J, Brum WS, Ennis GE, Kollmorgen G, Suridjan I, Langhough R, Jonaitis EM, Van Hulle CA, Betthauser TJ, Carlsson CM, Asthana S, Ashton NJ, Johnson SC, Shaw LM, Blennow K, Andreasson U, Bendlin BB, Zetterberg H, ADNI Cohort. Prevalence and Clinical Implications of a β-Amyloid-Negative, Tau-Positive Cerebrospinal Fluid Biomarker Profile in Alzheimer Disease. JAMA Neurol. 2023 Jul 31;80(9):969-79. PubMed.
University of Kentucky
It seems like the amyloid-negative (A-) tau-medial temporal lobe-positive (TMTL+) phenotype overlaps very broadly with PART.
PART has a different natural history than Alzheimer’s disease neuropathologic change (ADNC). In PART, the pathology stops at Braak NFT stage IV, at worst. In clinical pathology studies, the (bad) action in ADNC is in Braak stages V and even more so, the most severe stage, VI. Somehow Aβ, or something that Aβ is a proxy for, acts as an accelerant to get tau pathology into the neocortex where it causes its more horrible damage, and is, apparently, auto-propagating at that point.
PART occurs to differing degrees in different people. A given 105-year-old may have Braak stage II PART (sub-clinical), whereas a different 85-year-old may have Braak stage IV PART, and be affected by that.
The evaluation of autopsy tissue is more sensitive than PET, and thus, in autopsy studies only around 20 percent of people beyond age 80 years at death are amyloid-free, whereas biomarker studies indicate that around 40-50 percent or more are A-. By this simple math, there is likely quite a large number of people with a modicum of Aβ that is not detected by PET.
P-tau181 is a work in progress and the biofluid biomarkers related to ADNC are a moving target, but a fascinating and all-important one. It would seem odd to me if p-tau181 were actually specific for amyloid, rather than p-tau. The specificity of this particular biomarker may not be identical in every person’s hands, and these parameters are working themselves out. This is mission-critical for the global efforts to thwart dementia in the clinic!
I agree with Drs. Landau and Mormino that many of these folks with PART also have LATE-NC! And, for any level of ADNC (none to severe), LATE-NC is associated with more severe cognitive impairments.
Finally, many people who start with PART develop Aβ plaques and the picture morphs into ADNC/AD, over years. Therefore, I might tweak the abstract’s final sentence: “... individuals with A− TMTL+ are not on a pathologic trajectory toward AD ...” by adding “... unless or until they, in fact, are."
Make a Comment
To make a comment you must login or register.