Synaptic Proteins in CSF: New Markers of Cognitive Decline?
Quick Links
Plasma tests for Aβ and phospho-tau may have stolen the show at this year’s Alzheimer’s Association International Conference, held July 14-18 in Los Angeles, but proteomic and synaptic markers were not that far behind. A neural pentraxin, NPTX2, emerged as a contender for a marker of synaptic function and cognition. Other researchers debuted an ELISA for the synaptic vesicle protein SV2A, and showed it can be measured in the cerebrospinal fluid. Unbiased proteomic analysis of the CSF turned up these and other markers, which could parse subtypes of Alzheimer’s disease or reflect a person’s susceptibility based on his or her polygenic risk score (see Part 12 of this series). “It was incredibly exciting to see the field moving on from Aβ to other markers that could be functional readouts,” noted Beth Stutzmann, Rosalind Franklin University, Chicago. “I think NPTX2, SV2A, and other synaptic markers could prove very valuable, since what we are really interested in is cognitive function,” she said.
- Synaptic proteins can be released into the CSF.
- Could they be markers of synaptic function?
- At AAIC, neuronal pentraxin and a synaptic vesicle protein emerged as potential candidates.
Pentraxins are a family of proteins that bind AMPA glutamate receptors on the cell surface. By controlling the receptors’ maturation and recycling, pentraxins regulate synaptic plasticity. Expressed primarily in parvalbumin-positive inhibitory neurons, they help control network excitability (see image below). Previously, in collaboration with Doug Galasko’s group at University of California, San Diego, researchers at Paul Worley’s lab at Johns Hopkins Medical School, Baltimore, reported that NPTX2 levels fall in the brains and cerebrospinal fluid of AD patients (Xiao et al., 2017). Because NPTX2 levels appeared normal in cognitively unimpaired people who had amyloid plaques and neurofibrillary tangles in their brains at autopsy, the researchers reasoned that the protein might track with cognition.

Pentraxins and Plasticity. Neuronal pentraxin 1 (NP1) and 2 (also called Narp) and neuronal pentraxin receptor (NPR) form complexes on the cell surface that regulate AMPA receptor recycling on the postsynapse. [Courtesy of Cho et al., 2017 and Neuron.]
Denis Smirnov in Galasko’s lab set out to test this. Again collaborating with the Worley lab and with ADx Neurosciences, Ghent, Belgium, Smirnov correlated a variety of CSF markers with change in cognitive performance among 46 people with AD and 57 with mild cognitive impairment. All were patients at the UCSD Alzheimer’s Disease Research Center. They had been tested repeatedly on the Clinical Dementia Rating scale sum of boxes (CDR-sb), the Mattis Dementia Rating Scale (DRS), and for immediate and delayed recall in the California Verbal Learning Test (CVLT). Smirnov tracked cognitive scores against baseline CSF levels of total tau, NPTX2, and the synaptic markers neurogranin and SNAP25.
Over three years, people with MCI or AD who had high CSF total tau declined faster on the DRS and CDR-sb than those whose tau was low. Similarly, high SNAP25 correlated with faster decline in delayed and immediate recall, while those in the high-neurogranin group declined faster only on delayed recall. Strikingly, people who had low levels of CSF NPTX2 declined faster on all four measures (see image below).
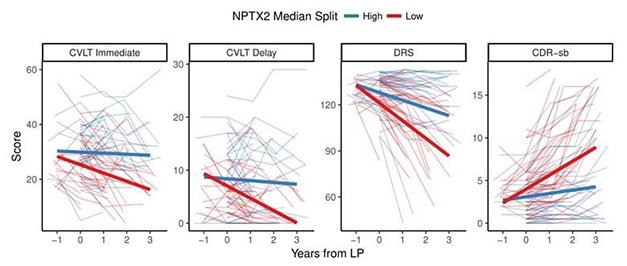
Predicting Cognitive Decline. People with low levels of NPTX2 in their CSF declined faster than those with higher levels. [Courtesy of Doug Galasko, UCSD.]
Smirnov repeated the analysis using ADNI data. He readily conceded that this was not a true replication because some fluid marker assays were different, and ADNI uses the ADAS-Cog rather than the DRS and the Rey AVLT instead of the CVLT. Still, a similar picture emerged. Among 80 people with AD dementia and 108 with MCI, low NPTX2 meant faster decline on all four cognitive measures. High neurogranin correlated with faster decline on the CVLT delayed-recall test; SNAP25 did not. ADNI also measures CSF levels of neurofilament light (NfL), a marker of neurodegeneration. Curiously, high CSF NfL did correlate with faster decline on the ADAS-Cog and CDR-sb, but less so than did low NPTX2, and NfL correlated with neither recall test.
Smirnov thinks that CSF NPTX2 could improve prediction of cognitive decline and disease progression based on other markers, such as Aβ and tau. In fact, survival analysis of the ADNI data indicated that people with MCI who have low CSF NPTX2 are three times more likely to develop dementia over five years.
In separate presentations, David Salmon, also from UCSD, and Eugeen Vanmechelen from ADx detailed how combinations of CSF markers improve diagnosis and prognosis. Analyzing data from 89 normal controls, 54 people with MCI, and 44 with mild AD, all from the UCSD ADRC, Salmon found that CSF NPTX2 alone predicted AD with an area under the curve (AUC) of 0.71, which was better than predictions based on SNAP25 or neurogranin. When Salmon combined markers, he got better results. The NPTX2/tau ratios identified AD patients with 94 percent accuracy (see image below); in fact, it distinguished AD from controls better than the Aβ42/tau ratio did. The NPTX2/tau ratio best predicted CVLT and DRS scores.
Vanmechelen analyzed CSF samples from 17 healthy controls, 36 patients with MCI due to AD, and 50 with AD, all from the Biobank of Institute Born-Bunge, Antwerp, Belgium. In this set, the NPTX2/tau ratio distinguished AD from controls with an AUC of 0.84 compared with 0.91 for Aβ42/tau. The NPTX2/tau ratio correlated with cognitive decline as judged by the mini-mental state exam.

CSF Predictors. Among synaptic markers, the NPTX2/tau ratio best predicted a diagnosis of dementia, with an AUC of 0.94. NPTX2 also popped up in unbiased proteomics of synaptosomes and CSF (see Part 12 of this series). [Courtesy of David Salmon, UCSD.]
Then there is synaptic vesicle glycoprotein 2A (SV2), another synaptic marker that has attracted interest of late. Most synaptic vesicles contain two to five copies of this transmembrane protein, which binds the PET ligand UCB-J. In brain-imaging studies, patients with epilepsy take up less of the ligand in affected brain areas, and AD patients bind less UCB-J in the hippocampus than do cognitively normal controls (Jul 2016 news; Aug 2018 conference news). Does SV2A end up in the CSF, and would levels there correlate with cognitive decline or AD? Nicholas Ashton, University of Gothenburg, Sweden, developed an immunoassay to find out.
Ashton tested a variety of antibodies against SV2A. One, which binds to an epitope on the N-terminal, gave a CSF signal. He tested the antibody in cohorts from Lund University, University of Gothenburg, and Paris Diderot Hospital. The Lund CSF samples came from 20 AD patients and 20 controls, UGothenburg had 42 AD patients and 50 controls, and Paris, 81 AD patients, 35 controls, 51 people with a different neurodegenerative disease, 30 people with MCI due to AD, and 49 with non-AD MCI.
Across all three cohorts, people with either clinically diagnosed AD or AD determined by biomarker criteria (Dubois 2014) had less SV2A in their CSF than controls did. So did FTD patients. However, people with Lewy body and vascular dementia seemed to have normal CSF SV2A levels.
Next Ashton correlated CSF SV2A against A/T/N staging scheme markers (Aug 2016 conference news; Jack et al., 2016). In the Lund and Paris cohorts, people whose CSF p-tau levels were above 80 pg/mL had less CSF SV2A regardless of Aβ positivity as determined by a cutoff of less than 550 pg/ml Aβ42. The data suggested that SV2A falls when p-tau rises. In agreement with this, when Ashton treated ATN markers as continuous variables, p-tau and total tau correlated with SV2A in both the AD patients and controls in both cohorts. Correlation with Aβ42 was weak. While CSF SV2A did correlate with neurogranin, SNAP25, GAP43, and synaptotagmin across the whole population, only the neurogranin correlation held up in AD patients. There was no correlation with NfL.
Scientists at AAIC were encouraged by the data, though with some niggling questions. How does this transmembrane protein get into the CSF? Why is it truncated? Ashton had shown Western blots indicating that SV2A in CSF appears slightly smaller than SV2A from solubilized brain extracts. He said he was trying to figure out the difference between the two using mass spec. Why did SV2A drop in FTD? Ashton does not know, but noted that those patients had no increase in CSF tau. “It’s possible SV2A reflects synaptic density in these patients,” he said.
Yet others wanted to know if Ashton had compared SV2A with NPTX2. He has not, but noted that NPTX2 levels correlate with other synaptic markers whereas SV2A does not, suggesting the latter might add predictive value. He did find that SV2A correlated with MMSE scores in AD patients. In short, Ashton believes that that SV2A might be a good CSF marker of synaptic impairment.—Tom Fagan
References
News Citations
- Next Up for Human Brain Imaging: Synaptic Density?
- PET Ligand Lights Up AAIC, May Detect Synapse Loss in AD
- Staging of Alzheimer’s, the Second: Neurodegeneration Does Not Equal Tauopathy
Paper Citations
- Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon D, Brewer J, Edland S, Wegiel J, Tycko B, Savonenko A, Reeves RH, Troncoso JC, McBain CJ, Galasko D, Worley PF. NPTX2 and cognitive dysfunction in Alzheimer's Disease. Elife. 2017 Mar 23;6 PubMed.
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014 Jun;13(6):614-29. PubMed.
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016 Aug 2;87(5):539-47. Epub 2016 Jul 1 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.