Stimulating Specific Neurons Mobilizes Dopamine-Depleted Mice
Quick Links
Textbook diagrams of the brain’s basal ganglia often represent their component nuclei as monolithic blocks, but a study in the May 8 Nature Neuroscience now shows how their finer substructures may harbor therapeutic opportunities. By stimulating distinct neuronal subpopulations in one component of the basal ganglia, the external globus pallidus (GPe), researchers led by Aryn Gittis at Carnegie Mellon University in Pittsburgh restored movement in a mouse model of Parkinson’s disease (PD).
“It’s a tour de force paper,” said Thomas Wichmann at Emory University in Atlanta. “The fascinating aspect is that they can manipulate cells specifically and get long-lasting anti-parkinsonism effects.”
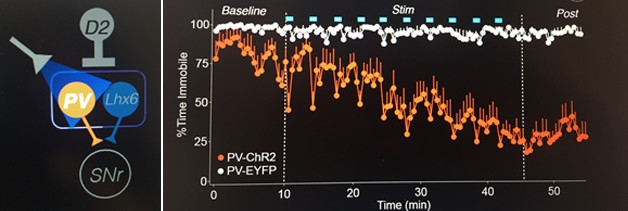
Mobility Regained: Activating parvalbumin-positive neurons in the GPe with optogenetics (left) improves mobility (orange trace) in dopamine-depleted mice (right). The effect persists even after light pulses (blue bars) stop. [Image courtesy of Aryn Gittis, Nature Neuroscience.]
The GPe helps suppress involuntary muscle contractions, and it has been implicated in the generation and amplification of pathological neural activity in PD (Mallet et al., 2008; Corbit et al., 2016). It silences downstream neurons in the substantia nigra pars reticulata, which in turn silence neurons in the motor thalamus. Essentially, the GPe puts the brakes on the brakes, preventing involuntary movements from interfering with voluntary ones.
Researchers predicted that stimulating the GPe might improve mobility in mouse models of PD, but that has not panned out. To understand why, Gittis and colleagues fine-tuned the stimulatory signals with optogenetics. First author Kevin Mastro generated mice expressing light-sensitive channelrhodopsin-2 in all cells of the GPe. He then injected the dopaminergic neuron killer 6-hydroxydopamine (6OHDA) into their medial forebrain and implanted optical fibers. Three to five days later, the researchers stimulated the GPe while the mice were free to move about. They saw essentially no effect: dopamine-depleted mice remained mostly immobile; when they did move, they were as slow as unstimulated controls.
“We thought maybe we needed to refine our stimulation protocol,” said Gittis. Researchers have long recognized the neuronal heterogeneity of the GPe, but have only recently begun to identify genetic markers that distinguish cell subpopulations (e.g., Mastro et al., 2014; Hernandez et al., 2015; Abdi et al., 2015). Mastro and colleagues focused on those distinguished by their expression of parvalbumin (PV-GPe neurons) and Lim homeobox 6 genes (Lhx6-GPe neurons).
Gittis started with PV-GPe cells, which are the most numerous. Using optogenetics to stimulate only PV-GPe cells, the researchers saw a drop in the percentage of time the mice stayed put (see image). Initially, the response was coupled to the light, but after a couple of pulses, the mice moved even when the light was off. By the 10th pulse, animals that had been immobile about 80 percent of the time were now on the move about 80 percent of the time. The effect persisted for several minutes after the last light pulse. The mice moved about fourfold faster and spent about the same amount of time walking, grooming, and executing other tasks as healthy mice.
To see how long the PV-ChR2 effect could last, the researchers left seven mice in a large box where they could roam freely and observed them for three hours after light stimulation. Remarkably, four remained active the entire time. However, they rarely stood up on their hind legs, they hunched their backs, and they moved with an irregular gait. Also, some of their movements seemed unnatural, possibly involuntary, noted Wichmann.
Nevertheless, stimulating the PV+ neurons evoked much more movement than stimulating the whole GPe. To probe why, the researchers recorded individual neurons. As expected, PV neurons fired more often following 30 light pulses. Intriguingly, many non-PV neurons fired less. The researchers hypothesized that the quiescent cells might be Lhx6-GPe neurons, which form synapses with the PV-GPe cells. They wondered if during global stimulation of the GPe, Lhx6-positive neurons might negate the effects of exciting PV-GPe cells, and if inhibiting Lhx6+ cells might also improve mobility.
Again, they used optogenetics to test this idea, expressing the light-sensitive proton pump archaerhodopsin (Arch) to silence synaptic activity in Lhx6-GPe cells. As expected, Arch-Lhx6 cells responded to flashes of light with sustained reductions in firing. The mice also began moving to a similar degree as when PV-ChR2 cells were stimulated. And when the light was turned off, Arch-Lhx6 mice continued to outrun controls for hours. Furthermore, neither silencing PV+ neurons nor stimulating Lhx6+ cells optogenetically had much effect on mobility. All told, the findings indicated that a combination of stimulated PV+ and quiescent Lhx6+ neurons stood the best chance of improving function.
Neither stimulating PV-ChR2 cells nor inhibiting Lhx6-Arch cells affected the average firing rates of SNr neurons, but it did reduce “bursting,” i.e., periods of rapid firing followed by quiescence. The fraction of highly bursting neurons dropped from 27 to 10 percent in PV-ChR2-stimulated mice and from 31 to 3 percent in Lhx6-Arch stimulated mice. Bursts diminished gradually and remained low for hours after light stimulation, mirroring the kinetics of the behavioral rescue. “We found a way to kick the system out of its dysfunctional state,” said Gittis. “We don’t know exactly how it happens yet, all we know is we’ve corrected a primary pathophysiology.”
Mark Bevan, Northwestern University, Chicago, has studied the plasticity of basal ganglia circuitry in response to dopamine depletion. He thinks neural circuitry has been changed. “It’s likely a change in synaptic connectivity in one or multiple sites,” he told Alzforum. “But it could also be a change in the intrinsic properties of the cells,” he added.
Gittis is eager to dissect the mechanism. “It looks like we’re driving plasticity,” she said. She wondered if the light treatment might rewire or repair the basal ganglia circuitry. “The ultimate goal would be to repair,” she said. Peter Tass at Stanford University thinks important mechanistic clues could emerge from studying the relationships between the different neuron activities in the GPe and SNr, including their correlation and synchronization. Wichmann suggested monitoring the subthalamic nucleus, which has reciprocal connections to the GPe.
Could the findings be used to develop new therapies for PD? Bevan believes that optogenetics and chemogenetics, which uses designer receptors that respond to an external ligand, might make their way into the clinic in the not-too-distant future, though Wichmann cautioned that many hurdles remain, such as achieving sufficiently high transfection rates in large brains.
Physicians already use deep-brain stimulation of the internal globus pallidus and the subthalamic nucleus to control movement in PD patients. Gittis thinks it may be possible to design a stimulation protocol that could predominantly stimulate PV+ cells.
However, Tass suggested it may be easier to shift SNr firing to a more favorable state without targeting particular GPe neurons. Indeed, he has devised a DBS protocol called coordinated reset, which coaxes neurons to unlearn pathological firing patterns. Using this technique in the subthalamic nucleus, Tass and others have reported anti-parkinsonism effects in a monkey model of PD that persisted for weeks, and they published a proof-of-concept study in humans (Tass et al., 2012; Wang et al., 2016; Adamchic et al., 2014). A study in patients with implanted electrodes is in the works.
Whether or not specifically manipulating GPe cells proves to be therapeutically valuable, the idea that there are sweet spots in the brain that can be used to manipulate disease is here to stay, says Bevan.—Marina Chicurel
References
Paper Citations
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008 Dec 24;28(52):14245-58. PubMed.
- Corbit VL, Whalen TC, Zitelli KT, Crilly SY, Rubin JE, Gittis AH. Pallidostriatal Projections Promote β Oscillations in a Dopamine-Depleted Biophysical Network Model. J Neurosci. 2016 May 18;36(20):5556-71. PubMed.
- Mastro KJ, Bouchard RS, Holt HA, Gittis AH. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J Neurosci. 2014 Feb 5;34(6):2087-99. PubMed.
- Hernández VM, Hegeman DJ, Cui Q, Kelver DA, Fiske MP, Glajch KE, Pitt JE, Huang TY, Justice NJ, Chan CS. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J Neurosci. 2015 Aug 26;35(34):11830-47. PubMed.
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci. 2015 Apr 29;35(17):6667-88. PubMed.
- Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, Meissner WG. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol. 2012 Nov;72(5):816-20. PubMed.
- Wang J, Nebeck S, Muralidharan A, Johnson MD, Vitek JL, Baker KB. Coordinated Reset Deep Brain Stimulation of Subthalamic Nucleus Produces Long-Lasting, Dose-Dependent Motor Improvements in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Non-Human Primate Model of Parkinsonism. Brain Stimul. 2016 Jul-Aug;9(4):609-17. Epub 2016 Mar 22 PubMed.
- Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, Silchenko A, Volkmann J, Deuschl G, Meissner WG, Maarouf M, Sturm V, Freund HJ, Tass PA. Coordinated reset neuromodulation for Parkinson's disease: proof-of-concept study. Mov Disord. 2014 Nov;29(13):1679-84. Epub 2014 Jun 28 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Mastro KJ, Zitelli KT, Willard AM, Leblanc KH, Kravitz AV, Gittis AH. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat Neurosci. 2017 May 8; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.