In People with African Ancestry, ApoE3 Variant Ups Alzheimer's Risk
Quick Links
Discovered in 1993, ApoE, the strongest risk gene for Alzheimer’s disease, was originally studied mostly in people of European ancestry. That has changed, and studies of non-European populations suggest that ancestry makes a difference. In the February 21 JAMA, a paper reports that among people of African descent, the R145C variant of ApoE3 nearly triples the risk of AD, but only among those who also carry a copy of ApoE4. Led by Yann Le Guen and Michael Greicius of Stanford University, the scientists suggest that this African-specific ApoE3 variant may unleash the worst of ApoE4 in this population. R145C contrasts other variants that may reduce AD risk among ApoE4 carriers of African descent. The findings highlight the importance of studying genetic risk across ancestries, and point to mechanisms that could protect against ApoE4.
- In ApoE3/E4 carriers, ApoE3-R145C variant nearly tripled AD risk.
- ApoE3[R145C]/ApoE4 carriers face equal odds as do ApoE4/E4 carriers.
- Also linked to African ancestry: Protective variants near the ApoE gene.
Regarding AD risk, ApoE4 packs less of a punch among people of African ancestry than it does among those of European or Asian descent. Myriad factors could influence this, but mounting evidence suggests that inherited DNA surrounding the ApoE gene, as opposed to elsewhere in the genome, softens the blow (Rajabli et al., 2018; Blue et al., 2019). This line of research expanded once geneticists looked beyond the three isoforms of ApoE, and searched for other variants in the gene, and indeed, the whole locus.
Le Guen and colleagues hunted for variants within the ApoE gene that affected AD risk among people with African ancestry. To do this, they scoured several databases housing whole-genome sequences, or imputed genotypes, from nearly 32,000 people with African ancestry, including almost 5,000 AD cases and more than 27,000 cognitively healthy controls. About 3 percent of samples came from Nigerians; the rest from African Americans.
The analysis unfolded in three stages, starting with a discovery cohort to identify variants, and followed by rounds of replication and validation. In short, they found that the R145C missense variant, which was always inherited as part of the ApoE3 allele, doubled to tripled the risk of AD among people with an ApoE3/E4 genotype. Approximately 4 percent of African descendants carry this ApoE3 variant, which is exceedingly rare among Europeans. Across all datasets, ApoE3 [R145C] lowered the age of AD onset by about five years among ApoE3/E4 carriers; in samples that came from longitudinal studies, the variant was tied to steeper cognitive decline. Interestingly, the ApoE3 [R145C] variant made no difference in the likelihood of someone's getting AD who had an ApoE3/E3 or ApoE2/E3 genotype. The ApoE3 [R145C]/ApoE4 combo hiked up AD risk to a similar extent as carrying two copies of ApoE4 (see image below).
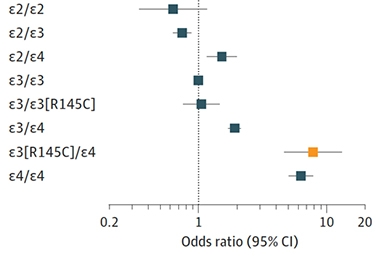
African Risk. In people of African ancestry, the R145C variant on the ApoE3 allele elevated AD risk, but only among those who co-inherited anApoE4. [Courtesy of Le Guen et al., JAMA, 2023]
Exactly how ApoE3 [R145C] raises risk is unclear. The mutation resides within ApoE's receptor binding domain. Previous studies have suggested that it weakens ApoE binding to all manner of receptors, including lipoprotein receptors and signaling receptors expressed on neurons (Rall et al., 1982; Ruiz et al., 2005). Le Guen found that this African variant also binds weakly to heparan sulfate proteoglycans, which are cell surface receptors involved in clearance and trafficking of Aβ and tau. Finally, previous studies have tied ApoE3 [R145C] to hyperlipidemia, suggesting that sluggish lipid metabolism could skew AD risk in carriers (Abou Ziki et al., 2014).
Could this variant explain why E4 seems a weaker risk factor among African descendants? That’s unlikely, given that [R145C] ApoE3 only crops up in about 4 percent of this population. However, recent studies have detected other variants that might attenuate ApoE4.
For example, researchers led by Jeffery Vance at the University of Miami in Florida identified genetic variants in European and Asian people that revved up the activity of enhancers near the ApoE promotor (Nuytemans et al., 2022). This could explain higher expression of ApoE4 in the brain among people with European ancestry as compared to non-Europeans (Griswold et al., 2021).
Subsequently, the Vance group identified yet another ancestry-specific variant in the vicinity of the ApoE gene (Rajabli et al., 2022). This single nucleotide polymorphism within a long noncoding RNA two megabases upstream of ApoE was predominantly found in people of African ancestry. In ApoE4/4 carriers it reduced the chances of getting AD about threefold. In the genotype-tissue expression (GTeX) database, which catalogues gene-expression differences throughout the body, this protective variant was linked to enhanced splicing of TMEM145 in the cerebellum. Little is known about the function of this transmembrane receptor, although its expression is reportedly elevated in the anterior cingulate cortex in people with dementia with Lewy bodies (Pietrzak et al., 2016). It remains unclear how this variant might stem ApoE4’s effect on AD risk.
Research on ancestral variants in and around ApoE—especially on how variants may interact with ApoE2, 3, and 4 in different populations—is in its infancy. Still, what's known so far suggests that variation in this locus modulates risk for Alzheimer’s disease.—Jessica Shugart
References
Mutations Citations
Paper Citations
- Rajabli F, Feliciano BE, Celis K, Hamilton-Nelson KL, Whitehead PL, Adams LD, Bussies PL, Manrique CP, Rodriguez A, Rodriguez V, Starks T, Byfield GE, Sierra Lopez CB, McCauley JL, Acosta H, Chinea A, Kunkle BW, Reitz C, Farrer LA, Schellenberg GD, Vardarajan BN, Vance JM, Cuccaro ML, Martin ER, Haines JL, Byrd GS, Beecham GW, Pericak-Vance MA. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 2018 Dec;14(12):e1007791. Epub 2018 Dec 5 PubMed.
- Blue EE, Horimoto AR, Mukherjee S, Wijsman EM, Thornton TA. Local ancestry at APOE modifies Alzheimer's disease risk in Caribbean Hispanics. Alzheimers Dement. 2019 Dec;15(12):1524-1532. Epub 2019 Oct 9 PubMed.
- Rall SC Jr, Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696-700. PubMed.
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005 Aug;46(8):1721-31. Epub 2005 May 1 PubMed.
- Abou Ziki MD, Strulovici-Barel Y, Hackett NR, Rodriguez-Flores JL, Mezey JG, Salit J, Radisch S, Hollmann C, Chouchane L, Malek J, Zirie MA, Jayyuosi A, Gotto AM Jr, Crystal RG. Prevalence of the apolipoprotein E Arg145Cys dyslipidemia at-risk polymorphism in African-derived populations. Am J Cardiol. 2014 Jan 15;113(2):302-8. Epub 2013 Oct 3 PubMed.
- Nuytemans K, Lipkin Vasquez M, Wang L, Van Booven D, Griswold AJ, Rajabli F, Celis K, Oron O, Hofmann N, Rolati S, Garcia-Serje C, Zhang S, Jin F, Argenziano M, Grant SF, Chesi A, Brown CD, Young JI, Dykxhoorn DM, Pericak-Vance MA, Vance JM. Identifying differential regulatory control of APOE ɛ4 on African versus European haplotypes as potential therapeutic targets. Alzheimers Dement. 2022 Jan 3; PubMed.
- Griswold AJ, Celis K, Bussies PL, Rajabli F, Whitehead PL, Hamilton-Nelson KL, Beecham GW, Dykxhoorn DM, Nuytemans K, Wang L, Gardner OK, Dorfsman DA, Bigio EH, Mesulam MM, Weintraub S, Geula C, Gearing M, McGrath-Martinez E, Dalgard CL, Scott WK, Haines JL, Pericak-Vance MA, Young JI, Vance JM. Increased APOE ε4 expression is associated with the difference in Alzheimer's disease risk from diverse ancestral backgrounds. Alzheimers Dement. 2021 Jul;17(7):1179-1188. Epub 2021 Feb 1 PubMed.
- Rajabli F, Beecham GW, Hendrie HC, Baiyewu O, Ogunniyi A, Gao S, Kushch NA, Lipkin-Vasquez M, Hamilton-Nelson KL, Young JI, Dykxhoorn DM, Nuytemans K, Kunkle BW, Wang L, Jin F, Liu X, Feliciano-Astacio BE, Alzheimer’s Disease Sequencing Project, Alzheimer’s Disease Genetic Consortium, Schellenberg GD, Dalgard CL, Griswold AJ, Byrd GS, Reitz C, Cuccaro ML, Haines JL, Pericak-Vance MA, Vance JM. A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer's Disease in African Ancestry. PLoS Genet. 2022 Jul;18(7):e1009977. Epub 2022 Jul 5 PubMed.
- Pietrzak M, Papp A, Curtis A, Handelman SK, Kataki M, Scharre DW, Rempala G, Sadee W. Gene expression profiling of brain samples from patients with Lewy body dementia. Biochem Biophys Res Commun. 2016 Oct 28;479(4):875-880. Epub 2016 Sep 22 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Le Guen Y, Raulin AC, Logue MW, Sherva R, Belloy ME, Eger SJ, Chen A, Kennedy G, Kuchenbecker L, O'Leary JP, Zhang R, Merritt VC, Panizzon MS, Hauger RL, Gaziano JM, Bu G, Thornton TA, Farrer LA, Napolioni V, He Z, Greicius MD. Association of African Ancestry-Specific APOE Missense Variant R145C With Risk of Alzheimer Disease. JAMA. 2023 Feb 21;329(7):551-560. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.