Paper Alert: CryoEM Structures of α-Synuclein Published
Quick Links
In the May 27 Nature, scientists publish the first-ever cryo-electron microscopy of α-synuclein fibrils. With resolution down to the atomic level, the analysis revealed two types of asymmetric fibril, each comprising two different protofibrils. The three-dimensional structures look nothing like those formed by Aβ or tau fibrils, or indeed by fibrils made in the lab from recombinant α-synuclein.
α-Synuclein structures will help scientists understand how the protein aggregates, and they will provide templates for designing PET ligands that can detect the fibrils in living people.
Michel Goedert, from the MRC Laboratory of Molecular Biology, Cambridge, U.K., and a senior author on the paper, presented the structures at the Tau2020 meeting in Washington, D.C., last March (Mar 2020 conference news).
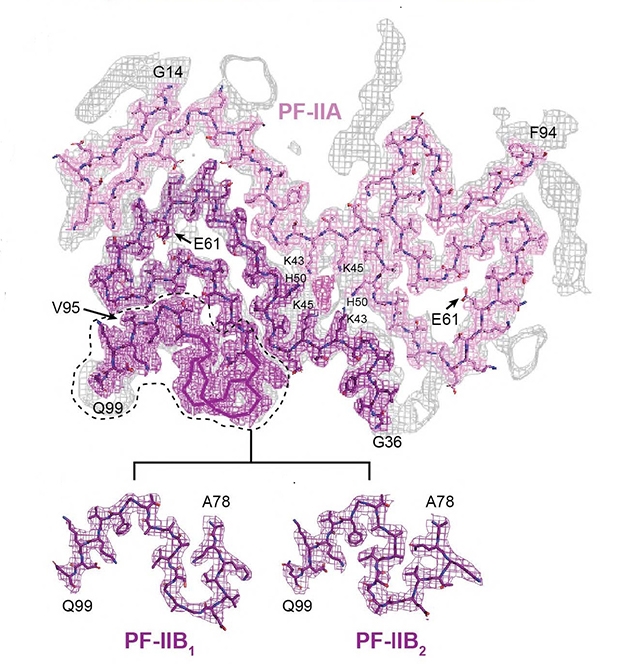
Synuclein Asymmetry. Two types of α-synuclein fibril were found in the brains of MSA patients. Type II (shown) comprises two types of protofilament. PF-IIA was similar to PF-IA in type I fibrils. The second protofilament came in two forms, PF-IIB1 and PF-IIB2. PF-IIB is smaller than the PF-IB of type I filaments. [Courtesy of Schweighauser et al., Nature.]
Goedert collaborated on the project with co-senior authors Sjors Scheres, also at the MRC, and Masato Hasegawa at the Tokyo Metropolitan Institute of Medical Science. Co-first authors Manuel Schweighauser and Yang Shi at the MRC isolated α-synuclein fibrils postmortem from five people who had died with multiple-system atrophy, a synucleinopathy that mostly attacks glial cells. Three of the volunteers had lived with the disease for about nine to 10 years, and they had mostly type I fibrils in their putamen. The other two lived with MSA for 18 and 19 years and had mostly type II fibrils in the same region. “This suggests that the duration of MSA may correlate with the ratio of filament types in putamen, but additional cases of disease are required to establish this more firmly,” note the authors.
The type of fibril differed by brain region, as well. Of three patients with predominantly type I filaments in the putamen, one had mostly type II in the cerebellum, while the other two had mostly type II in the cortex. It is unclear whether the two types are just as unequally distributed among nerve and glial cells, or how that relates to brain region.
Another surprising phenomenon is that, in the fibril cores (see image above), the protofibrils make much more extensive contact with each other than do protofibrils in Aβ or tau fibrils (Jul 2017 news; Sep 2017 news). Because the α-synuclein protofilament axis tilts with respect to the fibril axis, each protofilament monomer contacts three monomers in the opposing protofilament (see image below). How this affects aggregation dynamics and stability remains to be determined.

Protofibril Troika. Each synuclein protofilament monomer touches three in the opposing chain. The N terminus of the green PF-IB contacts the C terminus of the faded red PF-IA one up the chain. The C terminus of the green PF-IB binds the N terminus of the faded blue PF-IA one down the chain. [Courtesy of Schweighauser et al., 2020, Nature.]
Do similar fibrils explain other synucleinopathies, such as Parkinson’s and dementia with Lewy bodies? Apparently not. The authors also isolated fibrils from people who had DLB. These lacked the characteristic twist of MSA fibrils, which precluded cryoEM analysis. However, based on two-dimensional analysis, these structures are different.
For details on how the new structures relate to pathogenic mutations, and on a curious nonprotein cavity in the fibril cores, see our March conference news.—Tom Fagan
References
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B, Matsubara T, Tomita T, Ando T, Hasegawa K, Murayama S, Yoshida M, Hasegawa M, Scheres SH, Goedert M. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020 Sep;585(7825):464-469. Epub 2020 May 27 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Indiana University School of Medicine
Goedert’s group, including colleagues from Tokyo and Indiana, has made a seminal contribution to the field, and far beyond it. It is of particular note that “The three-dimensional structures look nothing like those formed by Aβ or tau fibrils, or indeed by fibrils made in the lab from recombinant α-synuclein.”
A common presumption we make is that recombinant protein samples, since they are “the same protein,” must behave like a native protein. This is a logical assumption. The authors, on the other hand, elegantly presented the first-ever cryo-electron microscopy of α-synuclein fibrils. Their paper revealed that their fundamental structure differed not only from tau or Aβ fibrils, which would not be unexpected, but that data amassed from recombinant α-synuclein is not reliable.
Our field is dominated by a reductionist hierarchism. Single mutations give rise to differences in protein or expression, which then give rise, in a yet unknown manner, to biochemical dysfunction, trivially leading to pathogenic bodies and diseases.
This group has once again demonstrated that emergent traits are often the rule in biology, where traits of a higher level of organization (structure) are not trivially deduced from the traits of a lower level. Or, indeed, even a simplified but “identical” (recombinant) example allegedly at the same hierarchical level does not trivially predict actual living behavior.
It is poetic that this larger-scale discovery was made using tools at some of the smallest scales (atomic-scale visualization). Passing the molecular baton to the atomic extent is joyful to watch. The precise twist and folding of protofibrillar structure are so consequential for brain and diseases (AD, PD, MSA). In a broad context, the finding is a historical leap.
Like neurodegenerative diseases, this finding evokes a parallel in how space exploration needs to be precise even at a large scale. We witnessed a successful docking of the SpaceX Dragon crew capsule at the International Space Station during the recent historic SpaceX/ NASA mission—what a convergence of astronaut and structural biologist of profound impact at very different levels!
Make a Comment
To make a comment you must login or register.