Paper Alert: Astrocytes Convert to Neurons (of the Dopaminergic Persuasion)
Quick Links
In the ultimate act of mouse astrocyte self-sacrifice, astrocytes leave their identities behind to transform into neurons and restore lost dopaminergic function in the brain. At least, they do so when prodded by scientists. In a paper published in Nature on June 24, researchers led by Xiang-Dong Fu at the University of California, San Diego, reported that dousing expression of a single protein—PTB—triggered the conversion of astrocytes into full-fledged neurons in the mouse brain. In the substantia nigra, some of these neuronal newbies pumped out dopamine and formed functional circuits with the neighboring striatum. In a mouse model of Parkinson’s disease, the converts restored dopamine and corrected motor deficits. The findings hint at the tantalizing possibility of conjuring brand-new neurons in people with neurodegenerative disease.
- Knocking down PTB converts astrocytes into neurons in the mouse brain.
- Some of the converts are dopaminergic, and form nigrastriatal circuits.
- In a Parkinson’s model, the new cells restore motor function.
Alzforum reported the bulk of the findings when Don Cleveland, UCSD, presented them at Keystone last year (Jul 2019 conference news). At the time, researchers at the conference asked myriad questions about the work, including whether the newly converted neurons formed functional circuits once instated. The published findings address some of these questions.
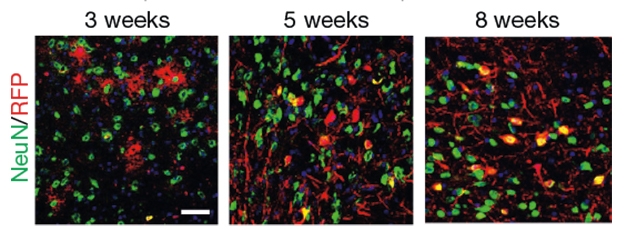
Gradual Conversion. After injection with AAV-shRNA-PTB, astrocytes infected with the virus (RFP, red) gradually transform into neurons (red + green = yellow). [Courtesy of Qian et al., Nature, 2020.]
Xu had previously reported that inhibition of pyrimidine tract binding (PTB) protein triggered cultured fibroblasts to differentiate into neurons (Jan 2013 news on Xue et al., 2013). The new study goes further, both by converting astrocytes into neurons, and by doing so in the brain. At Keystone, Cleveland described injecting mice directly into the substantia nigra with an adeno-associated virus that bears a short-hairpin RNA trained against PTB (shPTB). This converted astrocytes into neurons. Ten weeks later, 80 percent of the astrocytes infected with the virus had become neurons; a third of those were the dopaminergic kind.
In a crude mouse model of parkinsonism, in which scientists chemically ablate dopaminergic neurons on one side of the nigra with 6-OHDA, the converted cells corrected motor deficits. Cleveland reported that 6-OHDA ablation docked dopamine to about 25 percent of its normal level, and that neuronal conversion restored levels of the neurotransmitter up to 65 percent of normal.

Call It a Comeback? Dopaminergic neurons (green) are wiped out (middle) following lesion with 6-OHDA. New neurons (yellow) emerged following treatment with shPTB. [Courtesy of Qian et al., Nature, 2020.]
The published paper now adds evidence that the new nigral neurons built functional circuits with the striatum. In wild-type mice injected with the shPTB, first author Hao Qian and colleagues tracked the formation of axons projecting from the new neurons, which were fluorescently labeled. The number of fluorescent fibers increased over time, and by 12 weeks, the researchers detected an abundance of axons projecting from converted, dopaminergic neurons in the nigra into different regions of the striatum. They also spotted pre- and postsynaptic markers on the budding axons, suggesting the presence of synaptic connections.
The published paper now adds evidence that the new nigral neurons built functional circuits with the striatum. In wild-type mice injected with the shPTB, first author Hao Qian and colleagues tracked the formation of axons projecting from the new neurons, which were fluorescently labeled. The number of fluorescent fibers increased over time, and by 12 weeks, the researchers detected an abundance of axons projecting from converted, dopaminergic neurons in the nigra into different regions of the striatum. They also spotted pre- and postsynaptic markers on the budding axons, suggesting the presence of synaptic connections.
“This finding—together with the demonstration that the conversion process restored striatal dopamine levels and motor activity—provides evidence for a remarkable functional reconstitution of the nigrostriatal pathway by iDA neurons,” wrote Ernest Arenas of the Karolinska Institute in Stockholm in an accompanying editorial.

Rewiring the Nigrostriatal Circuit. Axonal fibers project from converted (red), dopaminergic (green, arrows) nigral neurons into the caudate putamen. [Courtesy of Qian et al., Nature, 2020.]
The scientists directly probed the functionality of the nigrostriatal circuit in living mice with electrophysiology. Using electrodes inserted into the medial temporal forebrain bundle to trigger dopamine release, and another electrode in the striatum to record the response, the researchers detected a dramatically diminished dopamine release on the injured side of the brain in 6-OHDA-lesioned mice. However, in three of four mice that underwent neuronal conversion, the circuit resumed near-normal firing patterns.
The researchers propose that a transient turn-down of PTB expression using antisense oligonucleotides (ASOs)—a therapeutically tractable approach—might efficiently convert human astrocytes into neurons. At Keystone, Cleveland reported that the ASO strategy worked in cultured cells, and the paper extends the finding to the mouse brain. Twelve weeks after injecting PTB-ASOs into one side of the substantia nigra, the researchers spotted new neurons thriving there, a fraction of which were dopaminergic. The ASO treatment also restored motor deficits in 6-OHDA-lesioned mice.
The published findings come on the heels of another neuronal conversion study, in which researchers used CRISPR to downregulate expression of PTB in the mouse striatum (Zhou et al., 2020). This approach also relieved motor deficits following 6-OHDA lesions.
Arenas referenced both papers. “The current studies promise to open a new chapter in the development of regenerative medicine for neurological disorders such as Parkinson’s disease,” he wrote in his editorial.—Jessica Shugart
References
News Citations
- Dopaminergic Neurons Conjured from Astrocytes Restore Motion
- Stem Cells: Simpler to Make, Easier on the Immune System
Paper Citations
- Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. Direct Conversion of Fibroblasts to Neurons by Reprogramming PTB-Regulated MicroRNA Circuits. Cell. 2013 Jan 9; PubMed.
- Zhou H, Su J, Hu X, Zhou C, Li H, Chen Z, Xiao Q, Wang B, Wu W, Sun Y, Zhou Y, Tang C, Liu F, Wang L, Feng C, Liu M, Li S, Zhang Y, Xu H, Yao H, Shi L, Yang H. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell. 2020 Apr 30;181(3):590-603.e16. Epub 2020 Apr 8 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Qian H, Kang X, Hu J, Zhang D, Liang Z, Meng F, Zhang X, Xue Y, Maimon R, Dowdy SF, Devaraj NK, Zhou Z, Mobley WC, Cleveland DW, Fu XD. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature, June 24, 2020
Annotate
To make an annotation you must Login or Register.

Comments
Harvard Medical School
This is a very interesting study from the perspective of trans-differentiation of mature cells into other cell types.
The data generated here depends heavily on the use of transgenic mice, introducing potential confounding factors such as promoter leakage. Cre-dependent animal models are very well-suited for developmental studies of cell lineage tracing, but are less appropriate for long-term studies in adult animals. Here the GFAP promoter will express high levels of Cre-recombinase in astrocytes and low levels in other cell types. Such low levels of Cre-recombinase activity over a period of months can lead to RFP labeling of mature neurons, and so there is a need for a robust reproduction of this data in non-transgenic mice.
The Parkinson lesion in the mice needs to use a dose of 6OHDA that gives a clear and complete loss of dopaminergic neurons rather than partial lesions (at low 6-OHDA doses used in this study). An interpretation consistent with the author’s data is a confound of two mechanisms rather than direct trans-differentiation into authentic midbrain dopaminergic neurons: (1) blocked ptbp1 turns transduced astrocytes into growth factor producing neuron-like cells, which can protect and activate remaining host dopamine neurons from the partial lesion, and (2) blocked btbp1 promotes Cre leakage, labeling non-lesioned neurons with RFP. Should the ptbp1 inhibition in vivo lead to neuroprotective events, or be shown to create authentic midbrain neurons in models with complete loss of dopamine neurons—this would be a very interesting preliminary study for evaluating therapeutic relevance.
Make a Comment
To make a comment you must login or register.