Panel of Blood Markers Signals Amyloid in Brain
Quick Links
Yet another potential blood test for amyloid pathology has entered the ring. Using mass spectrometry to take an unbiased look at plasma proteins, researchers led by Abdul Hye, King’s College London, found a panel of biomarkers that predicted with almost 90 percent accuracy whether cognitively normal people had a positive amyloid scan. Their work appears in the February 6 Science Advances. The study surfaced some proteins well-known from prior plasma research in Alzheimer’s disease—such as Aβ and neurofilament light proteins—and turned up some new ones, for example the transcription factor REST.
- Unbiased search for blood markers that correlate with brain amyloid.
- Mass spec identified panel of 10 proteins that was predictive.
- Broad-based replication will require simpler test platform.
“Aβ and NfL peptides were important in predicting AD, but alone they weren’t as predictable as when we combined them with novel proteins related to amyloid PET,” first author Nicholas Ashton told Alzforum. “Some are peripheral proteins, and we are not sure how they relate to amyloid pathology, so that is something that requires further investigation.” While the test is too technically demanding for routine clinical use, the candidates identified can for now be translated into ELISA-based, research-grade tests for wider use, Ashton said.
“Plasma biomarkers is a fast-moving field, and this novel study puts discovery proteomics in the spotlight,” said Douglas Galasko, University of California, San Diego. How some of the proteins identified get into plasma and from which cells is unclear, he added.
Suzanne Schindler, Washington University School of Medicine, St. Louis, considered the study exploratory, and preliminary. “They identified some interesting hits, but their results must be validated in a larger cohort,” she wrote to Alzforum.
While a pipe dream until a few years ago, plasma testing for Alzheimer’s pathology is now on the brink of becoming a reality, having benefited from more sensitive assays that suggest markers of Alzheimer’s pathology hide out in blood (Nov 2018 conference news). Most methods depend on detecting small changes in the ratio of Aβ42:40, which starts to decline in the plasma as amyloid accumulates in the brain. Ashton and colleagues wanted to take an untargeted approach to see if they could find other proteins in blood that correlated with amyloid pathology in the brain.
The authors tested blood samples from 144 people in the Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) cohort. According to PET scans, 100 had no detectable amyloid in the brain, while 44 did. After removing highly abundant proteins from the samples, such as albumin and immunoglobulins, the researchers digested the remainder into smaller peptides using trypsin and separated them by electric charge, dividing them into 24 fractions. They used mass spectrometry to identify and quantitate the contents of each fraction. They ended up measuring 2,356 proteins. A machine-learning algorithm then picked the combination of protein levels that best correlated with brain amyloid (see image below).
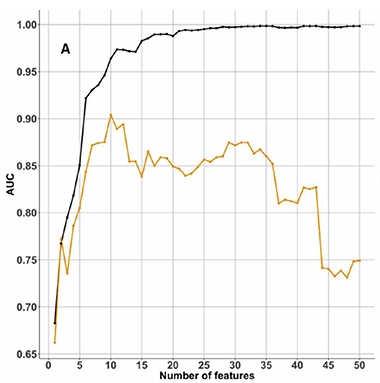
Pick the Best Combo. Machine learning tested which, and how many, proteins best correlated with brain amyloid. The area under the curves (AUC) for the AIBL training dataset (black) and the KARVIAH test dataset (orange) suggested an optimum of 10 proteins. [Courtesy of Ashton et al., 2019.]
This panel of 10 proteins associated most strongly with amyloid PET: prothrombin, Adhesion GPCR F4, APP, neurogenin-2 (NGN2), NfL, dynein heavy chain 10 (DNAH10), RE1-silencing transcription factor (REST), ribosomal protein S6 kinase alpha-3 (RPS6KA3), G protein–signaling modulator 2 (GPSM2), and forkhead-associated domain-containing protein 1 (FHAD1).
Next, the authors confirmed, again with mass spec, that this panel correlated with amyloid in an independent sample of cognitively normal participants in the Kerr Anglican Retirement Village Initiative in Ageing Health. KARVIAH is a Sydney-based cohort study established in 2016. It enrolled people 65 to 90 years of age who had subjective memory complaints but no dementia and lived in an assisted-living community. Of the 94 participants, 59 tested negative for amyloid, 35 positive. Accounting for age and ApoE status, the 10-protein panel predicted positive PET scans with 89 percent accuracy.
Ashton plans to reproduce these findings using ELISAs. That will make this panel test accessible to more labs and suitable for use in population-based screening. “It will be interesting to see a head-to-head comparison of this panel with Aβ42/40 in plasma across stages of amyloid-positive individuals,” wrote Galasko to Alzforum. “It may be analytically simpler to measure Aβ42 and Aβ40 as assays are refined, than a panel of 10 peptides plus APOE genotype.”
However, that’s not a given. “It’s hard to predict that a priori,” said Bruce Yankner, Harvard Medical School, Boston. “It’s unclear how well Aβ predicts disease onset, even with sensitive imaging measures,” he said. “Having some other measures that give a readout of damage to the neuron or some fundamental process that’s leading to the damage, might impart more prognostic specificity.” Yankner recently reported that REST is absent in neuronal nuclei derived from induced pluripotent stem cells of sporadic AD patients (Mar 2014 news; Feb 2019 news).
Schindler noted that AD biomarkers tend to be highly correlated with one another, so it may be that analysis of a subset of the plasma proteins identified here would predict just as well as the whole panel.—Gwyneth Dickey Zakaib
References
News Citations
Further Reading
Papers
- Hudd F, Shiel A, Harris M, Bowdler P, McCann B, Tsivos D, Wearn A, Knight M, Kauppinen R, Coulthard E, White P, Conway ME. Novel Blood Biomarkers that Correlate with Cognitive Performance and Hippocampal Volumetry: Potential for Early Diagnosis of Alzheimer's Disease. J Alzheimers Dis. 2019;67(3):931-947. PubMed.
- Liu S, Suzuki H, Ito H, Korenaga T, Akatsu H, Meno K, Uchida K. Serum levels of proteins involved in amyloid-β clearance are related to cognitive decline and neuroimaging changes in mild cognitive impairment. Alzheimers Dement (Amst). 2019 Dec;11:85-97. Epub 2019 Jan 12 PubMed.
Primary Papers
- Ashton NJ, Nevado-Holgado AJ, Barber IS, Lynham S, Gupta V, Chatterjee P, Goozee K, Hone E, Pedrini S, Blennow K, Schöll M, Zetterberg H, Ellis KA, Bush AI, Rowe CC, Villemagne VL, Ames D, Masters CL, Aarsland D, Powell J, Lovestone S, Martins R, Hye A. A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer's disease. Sci Adv. 2019 Feb;5(2):eaau7220. Epub 2019 Feb 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The Rockefeller University
The finding that decreased prothrombin was the highest-ranked feature in the Aβ+ cognitively unimpaired cohort is very intriguing. Thrombin derived from prothrombin is the last protease in the blood coagulation cascade. Aβ can activate Factor XII in the intrinsic coagulation pathway, which could ultimately lead to prothrombin depletion. This mechanism could partially explain the decrease in prothrombin observed in non-symptomatic Aβ-positive individuals.
References:
Zamolodchikov D, Chen ZL, Conti BA, Renné T, Strickland S. Activation of the factor XII-driven contact system in Alzheimer's disease patient and mouse model plasma. Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):4068-73. Epub 2015 Mar 16 PubMed.
Zamolodchikov D, Renné T, Strickland S. The Alzheimer's disease peptide β-amyloid promotes thrombin generation through activation of coagulation factor XII. J Thromb Haemost. 2016 May;14(5):995-1007. Epub 2016 Feb 29 PubMed.
Make a Comment
To make a comment you must login or register.