Mutant Tau Messes Up Connectivity of Newborn Neurons
Quick Links
Few studies have examined what happens to adult-born neurons in frontotemporal dementia. In the May 27 Journal of Neuroscience, researchers led by María Llorens-Martín at the Universidad Autónoma de Madrid report that in a mouse model of the disease, neurons born in the dentate gyrus of the adult hippocampus form aberrant connections. They extend short, simple processes that connect to a different set of cells than do newborn neurons in normal mice. The scientists also found more inhibitory synapses, and fewer excitatory synapses on these fledgling transgenic neurons. Similar anatomical and synaptic changes were apparent in postmortem hippocampal samples from people who had died with FTD. “This is the first evidence that this population of granule cells is altered in frontotemporal dementia,” Llorens-Martín told Alzforum.
- In mice carrying three tau mutations, newborn neurons form abnormal connections.
- Inhibitory synapses were more numerous, excitatory connections scarcer.
- Environmental enrichment partially rescued this phenotype.
Gerd Kempermann at the Center for Regenerative Therapies in Dresden, Germany, considers the findings important. “They highlight that in neurodegenerative disease, plasticity could be impaired at the level of cells, not just synapses,” he wrote to Alzforum. Orly Lazarov at the University of Illinois, Chicago, was impressed by the morphological analysis. She noted that the demonstration of a deficit at later stages of neurogenesis, when newborn cells are integrating into neuronal circuitry, is new and interesting. “This lays the groundwork for further studies looking into neurogenesis in FTD,” she said.
Previous studies by Llorens-Martín and others had reported less proliferation of neural precursor cells in mice carrying mutant human tau, resulting in fewer new neurons in hippocampus (Llorens-Martín et al., 2011; Komuro et al., 2015). To investigate what happens to these cells, first author Julia Terreros-Roncal used TauVLW mice. These carry human tau that has three mutations associated with FTD: G272V, P301L, and R406W. The mice develop prefibrillar tau aggregates and are believed to model FTD with parkinsonism linked to chromosome 17 (Lim et al., 2001).

Failure to Launch. In a mouse with mutant human tau (right), 4-week-old neurons (red) sprout shorter, simpler processes than in wild-type brain (left). [Courtesy of Terreros-Roncal et al., Journal of Neuroscience.]
When the mice were seven, 11, or 13 weeks old, Terreros-Roncal and colleagues injected retroviruses encoding different fluorescent markers into the dentate gyri. Retroviruses only integrate their genome into the DNA of dividing cells, and thus mark newborn neurons. In this way, the markers served as time stamps for neurogenesis. When the mice were 15 weeks old, new neurons in the TauVLW mice that were born four and eight weeks ago looked quite different from age-matched cells in wild-type mice. Their processes were shorter, simpler, less branched, and tended to sport more than one apical dendrite (see image above). Two-week-old neurons were only slightly different between TauVLW and wild-type mice.
In separate experiments, the authors examined the connections between the newborn cells and other neurons. They injected mice with retroviruses carrying genes for fluorescent forms of PSD95 and synaptophysin, markers of post- and pre-synapses, respectively. These labeled newborn neurons. TauVLW mice had fewer post-synapses in newborn dentate gyrus neurons, and smaller presynaptic terminals in hippocampal CA2 and CA3, the regions innervated by these cells.
Not only did newborn neurons in TauVLW mice have fewer connections than those in wild-type, but those connections were different. Markers of inhibitory synapses almost doubled, while markers of excitatory synapses were scarce. To identify connecting cells, the researchers injected fluorescently labeled rabies virus into the dentate gyrus. This virus is retrogradely transported, allowing researchers to visualize cells that synapsed onto newborn neurons. In wild-type mice, 20 percent of newborn neuron connections were with local inhibitory interneurons, and 49 percent were with excitatory neurons. In TauVLW mice, this percentage was almost reversed, with 61 and 22 percent inhibitory and excitatory, respectively. In keeping with this, only half as many newborn neurons in TauVLW mice expressed the activation marker early growth response protein 1. Moreover, TauVLW mice had twice as many neuropeptide Y-positive inhibitory interneurons in the dentate gyrus.
On top of the excitatory/inhibitory imbalance, dentate gyrus neurons in TauVLW mice made far fewer long-distance connections. Only 2 percent of their connections extended beyond the hippocampus, compared with 20 percent in wild-type mice.
Could these deficits be reversed? Scientists have shown that, in mice, decking out lab cages with toys and exercise wheels boosts neurogenesis. Would this also help newborn neurons in TauVLW mice? Apparently so. Eight weeks of this kind of environmental enrichment, starting at 7 weeks of age, boosted neurogenesis to levels seen in controls. These new neurons looked more normal. Their process length, branching, and apical dendrites were indistinguishable from those of control mice. Connectivity improved, with the number of post-synapses being nearly normal, and presynaptic size restored in the CA3 though not the CA2.
It is unclear how enrichment would restore neuronal morphology. Llorens-Martín noted that exercise is known to boost the level of growth factors such as BDNF, which profoundly affect axon extension and neuronal connections.
The authors found similar benefits when they artificially activated TauVLW newborn neurons after introducing a modified human muscarinic acetylcholine receptor via retrovirus. When the researchers stimulated this receptor, process length and arrangement came to resemble that of normal newborn neurons, while synaptic connections partially normalized. The treatment raised the number of the more mature mushroom spines, but did not rescue overall synaptic spine density.
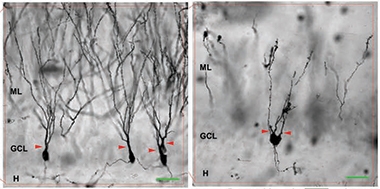
Stunted Growth. Dentate gyrus neurons in the FTD brain (right) have short, simple processes compared to healthy brain (left). [Courtesy of Terreros-Roncal et al., Journal of Neuroscience.]
Do findings in a triple-transgenic mouse model have any bearing on FTD? People with the disease do not have multiple mutations in tau, after all. To get a first sense, the authors analyzed postmortem hippocampal samples from three people with FTD and five controls. Silver staining revealed that dentate gyrus neurons in patients sprout more branches near the cell body than do neurons from controls, and fewer farther away, resulting in a stunted, simplified appearance. In healthy people, these neurons extend long processes with complex dendritic arbors (see image above). In FTD brain, total dendrite length was shorter, and some cells had more than one apical dendrite, just as in TauVLW mice. Likewise, immunostaining detected more inhibitory synaptic markers in FTD patients, ranging from 1.5- to fourfold more than in age-matched healthy controls. In these postmortem samples, the researchers could not tell if the neurons were recently born or decades old.
Llorens-Martín and colleagues will next study cultured hippocampal neurons to parse out how mutant tau may cause these defects. It remains to be seen whether similar deficits crop up in other tauopathies. Lazarov noted that the symptoms of behavioral variant FTD, where tau deposits mostly in the frontal lobe, are distinct from those seen in Alzheimer’s disease. “It would be interesting to know how these deficits and alterations in the maturation of new neurons contribute to the particular behavioral and cognitive alterations we see in FTD. We need more studies examining neurogenesis in this disorder,” Lazarov said.—Madolyn Bowman Rogers
References
Paper Citations
- Llorens-Martin M, Hernandez F, Avila J. Expression of frontotemporal dementia with parkinsonism associated to chromosome 17 tau induces specific degeneration of the ventral dentate gyrus and depressive-like behavior in mice. Neuroscience. 2011 Nov 24;196:215-27. PubMed.
- Komuro Y, Xu G, Bhaskar K, Lamb BT. Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol Aging. 2015 Jun;36(6):2034-42. Epub 2015 Mar 9 PubMed.
- Lim F, Hernández F, Lucas JJ, Gómez-Ramos P, Morán MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Mol Cell Neurosci. 2001 Dec;18(6):702-14. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Terreros-Roncal J, Flor-García M, Moreno-Jiménez EP, Pallas-Bazarra N, Rábano A, Sah N, van Praag H, Giacomini D, Schinder AF, Ávila J, Llorens-Martín M. Activity-Dependent Reconnection of Adult-Born Dentate Granule Cells in a Mouse Model of Frontotemporal Dementia. J Neurosci. 2019 Jul 17;39(29):5794-5815. Epub 2019 May 27 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.