Is Klotho’s Partner FGF23 a Cognition Protein?
Quick Links
The longevity gene klotho keeps memory keen, but how? New research raises the possibility that it might influence cognition through its signaling partners FGF23 and the fibroblast growth factor (FGF) receptor. In the March 7 eNeuro, Gwendalyn King and colleagues at the University of Alabama, Birmingham, reported that mice lacking FGF23 have memory problems similar to those seen in klotho knockouts. Meanwhile, researchers led by Emer McGrath at Brigham and Women’s Hospital, Boston, and Sudha Seshadri at Boston University report that older people with high levels of FGF23 in their blood are at elevated risk of developing dementia. Those findings appeared in the March 4 PLoS One. However, changes in circulating FGF23 levels are also known to impair kidney function. Kidney disease by itself can harm cognition, leaving it unclear if FGF23 acts directly in the brain or indirectly in the periphery.
- Mice lacking FGF23 have defective learning, memory.
- Some people with high serum FGF23 are at risk of dementia.
- This may be secondary to poor kidney health.
- Klotho longevity variant protects against ApoE.
“Both these papers give some hints that the FGF-23/klotho axis may be important to normal cognition, but significant follow-up work is still needed to clarify their exact roles,” noted David Drew at Tufts Medical Center in Boston. Drew also studies the relationship of FGF23 and klotho to cognitive function in humans, but was not involved in this research.
Klotho levels dwindle with age, and drop off steeply with disease progression in mouse models of amyloidosis (Dec 2007 conference news). Conversely, high levels of klotho boost cognitive performance in people, and memory in mice (May 2014 news; Aug 2017 news; Nov 2017 news).

FGF23 Linked to AD? People with the highest serum levels of the hormone (purple line) are twice as likely to develop AD over a dozen years as those with the lowest levels (blue line). [Courtesy of McGrath et al.]
A recent study in the March 13 Neurology adds genetic evidence for klotho’s protective effect in the brain. Researchers led by Ozioma Okonkwo at the University of Wisconsin, Madison, found that the klotho variant associated with high expression and longevity seemed to negate some effects of the ApoE4 allele. Among 82 people with this protective klotho variant, ApoE4 carriers accumulated no more brain amyloid than noncarriers. The mechanism is unclear. “Replication of these findings in larger, more diverse cohorts is warranted … Still, the current results are promising,” Argonde van Harten at VU University, Amsterdam, wrote in an accompanying editorial.
Researchers have elucidated at least one signaling pathway through which klotho acts. Klotho spans the cell membrane, where it binds FGF receptors to form a pocket that captures extracellular FGF, triggering intracellular signaling (Kurosu et al., 2006; Lee et al., 2018; Chen et al., 2018). It is unknown what effect this has on the brain. In the kidney, the binding of klotho and FGF23 stimulates excretion of phosphate and vitamin D, hence functions in their homeostasis (Shimada et al., 2004).
FGF23 levels appear to be tightly regulated. Mice with too much of it develop the vitamin-D-deficient bone disease rickets. Those with too little accumulate mineral deposits throughout their bodies (see image below) and age faster, as do klotho knockouts (Shimada et al., 2004; Sitara et al., 2004). FGFs form a large family and can regulate diverse cellular processes, however; hence FGF23 may play additional roles in other tissues.
King and colleagues investigated whether FGF23 knockout mice share the cognitive deficits of klotho knockouts. First author Ann Laszczyk found that 3-week-old FGF23 knockouts still performed like wild-type, but by 5 weeks of age, the knockouts had trouble remembering objects they had seen before, and forgot conditions that led to an electric shock. These hippocampal-dependent cognitive deficits matched those seen in klotho knockouts, but in other aspects the two mouse lines differed. Mice lacking klotho have enhanced synaptic plasticity and impaired neurogenesis, whereas mice lacking FGF23 were normal in both.
Were the learning defects of the FGF23 knockouts due to FGF23 in the brain? Probably not, King told Alzforum. The authors searched intensively for FGF23 mRNA or protein in wild-type mouse brain, but were unable to find significant expression. “That surprised us,” King said. Because of this, she believes that brain klotho acts via other mechanisms, independent of FGF23.
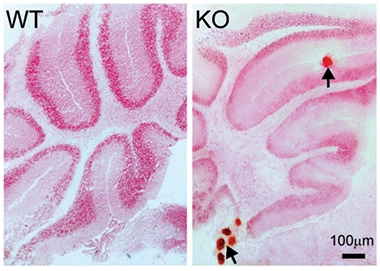
Calcified Brain. Mice lacking FGF23 (right) develop calcium deposits (red dots) in cerebellum and other body tissues, unlike wild-type mice (left). [Courtesy of Laszczyk et al.]
“We think the memory impairment we observed is most likely a matter of peripheral illness,” King said. The FGF23 knockouts develop kidney disease, grow slowly, and die by 9 weeks of age. Kidney problems are linked to cognitive deficits (Slickers et al., 2007; Sarnak et al., 2013; Hartmann et al., 2015). In particular, high FGF23 levels predict progression of kidney disease and worse cognition (Fliser et al., 2007; Drew et al., 2014). In future work, King plans to test this hypothesis by feeding FGF23 knockouts a low-phosphate diet, and assessing whether that preserves cognition.
For their part, Seshadri and colleagues were intrigued by the association of FGF23 with poor cognition in dialysis patients. They wondered whether serum FGF23 could predict cognitive outcomes in the general population as well. McGrath analyzed data from 1,537 participants in the Framingham cohort whose average age was 69. All were free of dementia at baseline, but over 12 years of follow-up, 122 people, or 8 percent of the cohort, developed it. Three-fourths of those cases were deemed to be AD.
Higher serum FGF23 correlated with dementia risk, whereby people in the highest quartile had 1.75 times the risk of dementia, and 2.1 times the risk of AD, as those in the lowest quartile. The relationship held after adjusting for the usual confounders, including sex, age, education, ApoE genotype, blood pressure, and cardiovascular disease. However, FGF23 levels did not associate with baseline cognitive performance, and longitudinal data on cognitive change was not available.
“It is puzzling that FGF23 does not associate with other cognitive measures as it does with dementia,” Jonas Mengel-From at the University of Southern Denmark, Odense, wrote to Alzforum (full comment below).
Were the cognitive problems simply a result of kidney disease? McGrath et al. noted that adjusting for kidney disease did not change risk, and suggested that FGF23 could be affecting klotho signaling in the brain. High serum FGF23 has been linked to low klotho (Hu et al., 2013; Shardell et al., 2016). However, the Framingham study did not measure serum klotho, so they could not determine if that relationship held here.
In addition, Drew noted that kidney function itself affects FGF23 levels. “I would not have waited until the final model to adjust for this likely confounder,” he wrote to Alzforum. He believes there is not yet convincing data that FGF23 directly affects cognition.
So far, FGF23 has not popped up in screens as a cognitive biomarker. Johan Gobom at the University of Gothenburg, Sweden, has done a large exploratory study of proteins and peptides in cerebrospinal fluid that associate with Alzheimer’s disease. He told Alzforum he does not see FGF23 in his dataset. Other AD fluid biomarker labs also noted that FGF23 has not come up in their work.
King believes the take-home message from these studies is the importance of kidney function for cognition. “You need to have a healthy body if you want the lowest risk of developing memory impairment,” she wrote.—Madolyn Bowman Rogers
References
News Citations
- San Diego: Klotho Spins Threads Linking Aging and AD Research
- Longevity Gene Boosts Brainpower, Even in the Young
- Shot of Klotho Boosts Memory In Aging and Diseased Mice
- Brain-Specific Klotho Isoform Fortifies Memory
Paper Citations
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006 Mar 10;281(10):6120-3. Epub 2006 Jan 25 PubMed.
- Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J. Structures of β-klotho reveal a 'zip code'-like mechanism for endocrine FGF signalling. Nature. 2018 Jan 25;553(7689):501-505. Epub 2018 Jan 17 PubMed.
- Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018 Jan 25;553(7689):461-466. Epub 2018 Jan 17 PubMed.
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004 Feb;113(4):561-8. PubMed.
- Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004 Feb 6;314(2):409-14. PubMed.
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004 Nov;23(7):421-32. PubMed.
- Slickers J, Duquette P, Hooper S, Gipson D. Clinical predictors of neurocognitive deficits in children with chronic kidney disease. Pediatr Nephrol. 2007 Apr;22(4):565-72. Epub 2006 Dec 16 PubMed.
- Sarnak MJ, Tighiouart H, Scott TM, Lou KV, Sorensen EP, Giang LM, Drew DA, Shaffi K, Strom JA, Singh AK, Weiner DE. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013 Jan 29;80(5):471-80. Epub 2013 Jan 9 PubMed.
- Hartmann H, Hawellek N, Wedekin M, Vogel C, Das AM, Balonwu K, Ehrich JH, Haffner D, Pape L. Early kidney transplantation improves neurocognitive outcome in patients with severe congenital chronic kidney disease. Transpl Int. 2015 Apr;28(4):429-36. Epub 2015 Jan 13 PubMed.
- Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, MMKD Study Group, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007 Sep;18(9):2600-8. Epub 2007 Jul 26 PubMed.
- Drew DA, Tighiouart H, Scott TM, Lou KV, Fan L, Shaffi K, Weiner DE, Sarnak MJ. FGF-23 and cognitive performance in hemodialysis patients. Hemodial Int. 2014 Jan;18(1):78-86. Epub 2013 Oct 24 PubMed.
- Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503-33. PubMed.
- Shardell M, Semba RD, Rosano C, Kalyani RR, Bandinelli S, Chia CW, Ferrucci L. Plasma Klotho and Cognitive Decline in Older Adults: Findings From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2016 May;71(5):677-82. Epub 2015 Aug 21 PubMed.
Further Reading
Primary Papers
- Laszczyk AM, Nettles D, Pollock TA, Fox S, Garcia ML, Wang J, Quarles LD, King GD. FGF-23-deficiency impairs hippocampal-dependent cognitive function.
- McGrath ER, Himali JJ, Levy D, Conner SC, Pase MP, Abraham CR, Courchesne P, Satizabal CL, Vasan RS, Beiser AS, Seshadri S. Circulating fibroblast growth factor 23 levels and incident dementia: The Framingham heart study. PLoS One. 2019;14(3):e0213321. Epub 2019 Mar 4 PubMed.
- Erickson CM, Schultz SA, Oh JM, Darst BF, Ma Y, Norton D, Betthauser T, Gallagher CL, Carlsson CM, Bendlin BB, Asthana S, Hermann BP, Sager MA, Blennow K, Zetterberg H, Engelman CD, Christian BT, Johnson SC, Dubal DB, Okonkwo OC. KLOTHO heterozygosity attenuates APOE4-related amyloid burden in preclinical AD. Neurology. 2019 Apr 16;92(16):e1878-e1889. Epub 2019 Mar 13 PubMed.
- van Harten AC. Comment: Longevity gene KLOTHO may play a role in Alzheimer disease. Neurology. 2019 Apr 16;92(16):751. Epub 2019 Mar 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
ADvantage
McGrath et al. report results from a 12-year follow-up of a Framingham Heart Study population, suggesting that a high serum FGF23 level is correlated with dementia. The higher serum FGF23 was associated with an increased risk of incident dementia and AD, but was not associated with structural brain measures predictive of vascular brain injury or with performance on neurocognitive testing.
Klotho is an anti-aging protein that serves as a co-receptor with the FGF receptor to facilitate FGF23 signaling in the kidney resulting in calcium, phosphate, and vitamin D homeostasis. The connection between Klotho and FGF23 was made when it was realized that Klotho-deficient and FGF23-deficient mice share similar, albeit not identical, phenotypes.
In a second article connecting FGF23, Klotho, and cognition, Laszczyk and colleagues show that FGF23 deficiency also leads to hippocampus-dependent cognitive impairment, like that seen in Klotho-deficient mice. However, FGF23-deficient brains had no change in hippocampal synaptic plasticity, no gross structural or developmental defects, and only minor impairment to postnatal hippocampal neurogenesis, in contrast to Klotho-deficient mice.
The authors hypothesize that hippocampal-dependent cognitive impairment could be the result of an increasingly toxic brain micro-environment caused by FGF23 deficiency and a resulting lack of normal ion homeostasis. They conclude that although both Klotho- and FGF23-deficient mice suffer from cognitive decline, in the brain this may occur in parallel pathways and not via Klotho-dependent FGF23 signaling, as demonstrated in the kidney. Interestingly, it was shown by others that patients with severe kidney disease suffer from cognitive decline, suggesting that some peripheral factor may affect the brain.
Nevertheless, Hensel et al. (2016) provided evidence that FGF23 circulates in CSF and that primary hippocampal cells do respond to recombinant FGF23 by signaling that affects neuronal morphology and synaptic density. Thus, FGF23 may serve as a ligand for FGF-R and Klotho in the brain. However, these results need confirmation by other groups.
A recent review in Frontiers in Endocrinology describes Klotho-dependent and independent actions of FGF23. FGF23 also signals directly via the FGF receptor in tissues lacking Klotho, including induction of cardiac hypertrophy and fibrosis, elevation of inflammatory cytokine expression in the liver, and inhibition of neutrophil recruitment (Richter and Faul, 2018).
References:
Hensel N, Schön A, Konen T, Lübben V, Förthmann B, Baron O, Grothe C, Leifheit-Nestler M, Claus P, Haffner D. Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem. 2016 Jun;137(5):756-69. Epub 2016 Mar 7 PubMed.
Richter B, Faul C. FGF23 Actions on Target Tissues-With and Without Klotho. Front Endocrinol (Lausanne). 2018;9:189. Epub 2018 May 2 PubMed.
University of Southern Denmark
Both Laszczyk et al. and McGrath et al. find that FGF23 is related to cognitive performance, that being in both the mouse and the human. FGF23 is already known to play a role in mineral metabolism, a process acting across multiple organs. Specially, the study implicating serum FGF23 as a risk factor for dementia and Alzheimer’s disease is interesting and fits well with the pathway-related Klotho gene variation that also acts on cognitive performance. However, it is puzzling that FGF23 does not associate with other cognitive measures as it does with dementia. From studies of Klotho, e.g. genetic studies, the KL-VS variant is associated with normal cognitive abilities and is related to synaptic glutamate functions, which is a mechanism for normal learning abilities.
Klotho is associated with a broad range of maladies including cardiovascular diseases, kidney dysfunction, and cognitive disabilities. And klotho is fundamental in several organ functions, also in the brain. But as the associations are dependent on aging, it would be expected also to be the case for the earlier pathway component FGF23. In these studies, timing as well as dosage, i.e. level of FGF23 or Klotho, are important factors to consider, and may be a reason for the inconsistency across cognitive traits as also highlighted in the papers.
For background, appropriate levels of phosphate in the body are maintained by the coordinated regulation of the bone-derived growth factor FGF23 and the membrane-bound protein Klotho. The endocrine actions of FGF23, in association with parathyroid hormone and vitamin D, mobilize sodium–phosphate co-transporters that control renal phosphate transport in proximal tubular epithelial cells. The availability of an adequate amount of Klotho is essential for FGF23 to exert its phosphaturic effects in the kidney.
In the presence of Klotho, FGF23 activates downstream signaling components that influence the homeostasis of phosphate, whereas in the absence of this membrane protein, it is unable to exert such regulatory effects, as demonstrated convincingly in animal models. Several factors, including phosphate and vitamin D, can regulate the production of both FGF23 and Klotho and influence their functions.
Make a Comment
To make a comment you must login or register.