Deep Sleep Makes Waves for CSF
Quick Links
Sleep is profoundly restorative for the brain, partly because it clears solutes and waste products. This flushing works best during the deepest periods of sleep, but why is that? Data from the lab of Laura Lewis, Boston University, suggest that during stretches of slow-wave, non-REM sleep, periodic waves of cerebrospinal fluid (CSF) wash through the brain. Interestingly, the timing of these waves corresponds with the slow neuronal oscillations that define this stage of sleep, and with accompanying fluctuations in blood-oxygen level. The results suggest that electrophysiological waves drive slow pulses of CSF, and that this coupling is mediated by changes in blood volume.
- Neural oscillations during deep sleep cause fluctuations in blood flow.
- Blood flow changes in turn bring on coordinated waves of CSF.
- The results suggest that electrophysiological signals and CSF dynamics are linked.
“This is an exciting study,” said Henrik Zetterberg, University of Gothenburg, Sweden, who was not involved in the project. “It shows quite convincingly that a change in neuronal activity precedes the change in fluid movement in the brain in deep sleep,” he said.
Scientists know sleep is critical for brain health and memory consolidation (for a review, see Diekelmann and Born, 2010). They define deep sleep stages by the appearance of unique electrophysiological rhythms, in which neurons across the brain fire in slow synchrony with one another, at frequencies of 4Hz or below. These waves are crucial for memory consolidation. Could they be useful for other reasons, as well?
Toxic waste products such as Aβ clear out of the brain faster during sleep than waking hours, and this clearance is strongest during deep sleep (Xie et al., 2013; Hablitz et al., 2019). Lewis wondered if CSF dynamics were changing during sleep to enhance the clearance of solutes, and how those changes come about.
In the study, first author Nina Fultz and colleagues took brain images of young, healthy adults while they slept. They recruited 13 volunteers to sleep over at their lab. Late at night, participants donned an electroencephalography (EEG) cap and fell asleep inside an MRI scanner.
The scientists simultaneously monitored three things: the electrophysiological oscillations that propagate throughout the brain, the corresponding changes in blood-oxygen level, and the CSF flow in the brain. They measured brain waves with EEG, and blood oxygenation with functional MRI measuring blood-oxygen-level–dependent (BOLD) signal before and during sleep. They measured the velocity of CSF flow into the brain using a particularly high rate of fMRI image acquisition. CSF newly enters the brain through the fourth ventricle, seen in the lowest image slices, aka axial plane. There it appeared bright (see image below). This bright signal faded as the CSF moved up into the brain to higher slices. The speed signal arose because faster-flowing CSF stayed bright into higher slices, while slower-moving fluid appeared darker in lower slices.

CSF Speed Trap. CSF enters the brain at the fourth ventricle (red arrow), where the signal appears bright. The signal darkens as it moves farther into the brain. Depending on how fast the fluid moves, the signal appears brighter or darker in higher slices (yellow lines). [Courtesy of Science/AAAS.]
The first thing Fultz noticed was that shortly after deep sleep started, periodic waves of CSF pressed into the brain, at a rate of one wave every 20 seconds. This compares to smaller but faster four-second waves of CSF that move in while a person is awake. Curiously, these waves were timed both with blood dynamics—such that CSF rushed into the brain while blood ebbed away—and with electrophysiological activity.
Fluid Interchange. In deep sleep, pulses of blood oxygenation (red) alternate with waves of cerebrospinal fluid (blue), suggesting rhythmic drops in blood volume draw in CSF to the brain.
As the brain entered deep sleep, the neural oscillations changed first, followed a few seconds later by fluctuations in blood oxygenation. This was accompanied by alternating waves of CSF flow (see video above). All three measurements were precisely timed to one another, with each trough in EEG amplitude followed immediately by a drop in blood oxygenation, which was tightly coupled to an influx of CSF (see image below).
“It seems that the electrical neuronal rhythms your brain experiences during sleep are tightly coupled to these fluid dynamics,” Lewis told Alzforum.
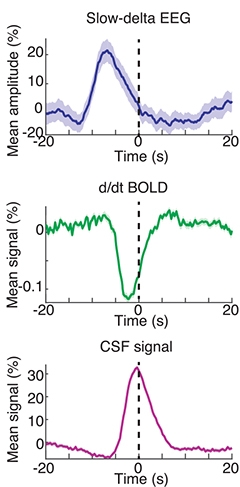
Phase Lock. When a person is in deep slumber, a drop in their EEG amplitude is accompanied by a lowering of the BOLD signal, which corresponds to an inrush of CSF to the brain (peak marked as time 0). [Courtesy of Science/AAAS.]
How could these seemingly independent factors be so entrained? To try to explain this, the researchers generated a computational model. When neurons are less active, they need less blood, so blood volume likely decreases. Because the intracranial volume stays constant, this decrease creates room for CSF to flood in. “The rhythms we saw looked a lot like what the model would predict based on this chain of events.” Lewis said. The peak of CSF flow occurred 6.4 seconds after a peak in slow-wave EEG. “That suggests a biophysical coupling. It’s a more holistic system than we realized.”
“From a functional neuroanatomy point of view, the study is extremely useful, as it provides evidence that CSF influx into the brain parenchyma is closely linked to blood flow and neuronal activity,” said Roxana Carare, University of Southampton, England, U.K.
Lewis said it would be interesting to test this mechanism in a mouse model and see how perturbations in any one factor affect the others. “Data suggest that people who don’t have the electrical rhythms typical of healthy sleep may also have altered CSF sleep dynamics,” said Lewis. “These are probably really important for various aspects of brain health.”
For instance, people with Aβ pathology have less slow-wave activity during non-REM sleep (Mander et al., 2015). If Lewis’ findings apply to the aging/AD population, it could mean fewer, smaller waves of CSF in patient populations, she suggested. However, this study was done in healthy young adults and did not examine clearance of solutes, and so cannot inform about clearance of disease proteins from the brain in AD, noted Erik Musiek, Washington University in St. Louis (comment below).
“It will be interesting to assess whether the CSF dynamics linked to SWS can be used as a biomarker for disease states, and whether strategies that restore SWS can rescue brain function in neurodegeneration,” wrote Søren Grubb and Martin Lauritzen of the University of Copenhagen, Denmark, in an accompanying editorial.
This mechanism may put people who are unable to enter deep sleep—for instance because of obstructive sleep apnea—at higher risk of a neurodegenerative disease, said Zetterberg. Lewis’ findings suggest a need for treatments that lengthen time in deep sleep for people who struggle to get enough, and an evaluation of sleep treatments based on their ability to increase the amount of deep sleep, rather than just total sleep time, he said.—Gwyneth Dickey Zakaib
References
Paper Citations
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010 Feb;11(2):114-26. Epub 2010 Jan 4 PubMed.
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013 Oct 18;342(6156):373-7. PubMed.
- Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019 Feb;5(2):eaav5447. Epub 2019 Feb 27 PubMed.
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015 Jul;18(7):1051-7. Epub 2015 Jun 1 PubMed.
Further Reading
News
- Do Brain Waves During Sleep Reflect Aβ and Tau Pathologies?
- Another Reason to Catch Some Zzzs: Sleep Regulates Tau Release
- New Ties between AD and the Stages, Waves, and Molecules of Sleep
- Disturbed Sleep Exerts Toll on Memory and Neurodegeneration
- Sleep and Brain Cleansing—Fresh Insights into Regulation and Disruption
- Does Amyloid Disturb the Slow Waves of Slumber—and Memory?
Primary Papers
- Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019 Nov 1;366(6465):628-631. PubMed.
- Grubb S, Lauritzen M. Deep sleep drives brain fluid oscillations. Science. 2019 Nov 1;366(6465):572-573. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
This is an exciting paper, which provides a possible mechanistic link between slow-wave neuronal activity during NREM sleep and pulsations in CSF flow. It offers a new insight into how sleep-related changes in neuronal activity potentially alter CSF dynamics via regulation of vascular tone. It will be interesting to see exactly how these findings integrate with glymphatic function and fluid flow through meningeal lymphatics. Interestingly, the Nedergaard lab and others have shown that arterial pulsation can drive interstitial fluid movement in the brain, and this paper suggests that that may occur as a result of changes in neuronal firing during sleep.
Despite my enthusiasm, it is important to first note that this was observed in a small number of healthy adults, and must be replicated by other groups. More importantly, any connection to Alzheimer's disease or Aβ clearance is strictly speculative, as no AD patients were examined in this study, and no measures of Aβ or other AD-related molecules were performed. The authors state this, and do speculate on this possibility briefly in the paper (which is appropriate), but many other media outlets are already reporting this finding as a breakthrough in our understanding of how the sleep clears toxins from the brain in AD. This study does not examine clearance of any substances from the brain, though it has clear implications for that process.
Make a Comment
To make a comment you must login or register.