Astroglial Markers Poised for Stardom?
Quick Links
When stuck with lemons, make lemonade. The “lemon” for Nobuyuki Okamura and colleagues at Tohoku University, Sendai, Japan, was THK-5351, a tracer they had developed to detect neurofibrillary tangles. Alas, the compound bound monoamine oxidase B, as well, souring its usefulness for tau PET. Now for the sweet pivot. Astrocytes predominantly produce MAO-B, and they squeeze out more of this enzyme when stressed, as during the astrogliosis that occurs in response to amyloid and tau pathology. What if a few tweaks to THK-5351 might turn it from a bad tau tracer into a good astrocytosis tracer? The field sure is thirsty for a good astrocytosis tracer.
- 18F-SMBT-1 binds monoamine oxidase B.
- It has the properties of a good PET tracer for astrogliosis.
- Astrocyte glial fibrillary acidic protein ticks up in blood of people with AD.
At this year’s ADPD meeting, which began online March 9, Victor Villemagne, who has moved to the University of Pittsburgh, Pennsylvania, reported that 18F-SMBT-1—just such a derivative—bound MAO-B in the human brain reversibly and with high specificity. In PET scans, people who tested positive for amyloid bound much more of the new ligand in their brains than did amyloid-negative controls. The upshot: Researchers may soon have a better PET tracer for astrogliosis to accompany those that already detect amyloid, neurofibrillary tangles, changes in glucose metabolism, and, perhaps, synapse number.
Care for a chaser with that lemonade? A blood test for astrogliosis may soon come your way as well. At ADPD, Andrea Benedet, University of Gothenburg, Sweden, reported that the concentration in plasma of glial fibrillary acidic protein—which is produced by astrocytes in the central nervous system—climbs twice as high in people who have AD than it does in age-matched controls. Plasma GFAP correlated with brain amyloid and tangles, though the latter association was primarily driven by amyloid, Benedet reported. Curiously, plasma GFAP tracked with amyloid more tightly than did its level in cerebrospinal fluid (CSF). “This is the first time we see a plasma biomarker performing better than a CSF biomarker in predicting amyloid,” said Benedet.
“It is surprising that GFAP seems more specific in plasma than in CSF, but we, too, see better correlation with plasma GFAP and Aβ in our cohorts,” Oskar Hansson, Lund University, Sweden, told Alzforum.
Recent studies led by Ralph Martins at Edith Cowan University, Joondalup, Western Australia, and Charlotte Teunissen at Vrije Universiteit, Amsterdam, support Benedet’s findings. They found that amyloid-positive volunteers had more GFAP in their plasma than did amyloid-negative controls, and that, when combined with the plasma Aβ2/40 ratio, GFAP improved predictions of amyloid positivity.
Astrogliosis PET
Okamura and colleagues used THK-5351 as a starting point to finesse PET ligands for MAO-B (see image below). As reported in the February Journal of Nuclear Medicine, first author Ryuichi Harada and colleagues removed an amino group that was essential for the compound to bind tangles (Harada et al., 2021). This simple tweak dramatically increased the compound’s affinity and specificity for the oxidase. In vitro, the dissociation constant for binding MAO-B was 3.7 nM for the new compound, dubbed SMBT-1. Binding to MAO-A was almost 200-fold weaker, with a Kd of 713 nM. Affinities for Aβ and tau aggregates from human brains were weaker still, with Kd’s of more than 1,000 nM, indicating that SMBT-1 specifically binds MAO-B.
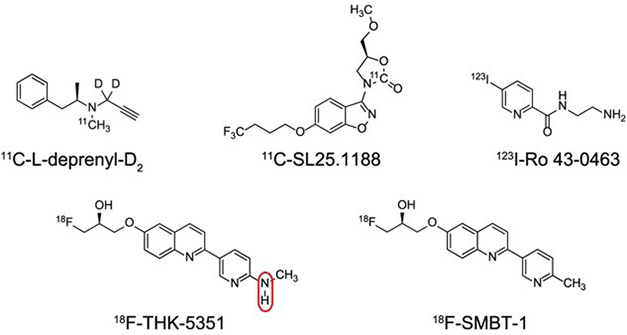
Lose that Amine. Clipping off an N-H group (circled) turned the experimental tau PET tracer THK-5351 into SMBT-1, a reversible and specific ligand for MAO-B. SMBT-1 has much better tracer properties that other MAO-B ligands (top row). [Courtesy of Harada et al., Journal of Nuclear Medicine, 2021.]
But would it work in human tissue? Using autoradiography, Harada and colleagues found that more SMBT-1 bound to sections from AD brains than control brains.
How about for imaging? After injection into the blood streams of mice, the compound rapidly flooded the brain and then quickly washed out—just what the tracer developer ordered.
This contrasts with deprenyl, the most widely used tracer for MAO-B (Feb 2012 news). Deprenyl ends up binding irreversibly to the oxidase when it forms a covalent bond with a flavin cofactor in the enzyme’s catalytic site. Even deuterating deprenyl to slow this chemical dalliance does not fully eliminate the covalent binding.

Voilá, Astrocytosis. Axial, coronal, and sagittal images showing more widespread retention of SMBT-1 in a person with AD (bottom) compared to an age-matched healthy control (top). [Courtesy of Victor Villemagne.]
Toxicity studies indicated that the teeny amounts of SMBT-1 needed for PET would be safe. Villemagne, Harada, and colleagues tested SMBT-1 in 82 participants in Australia’s AIBL longitudinal study. Ten were healthy young controls, 55 healthy old controls, 12 were old with mild cognitive impairment, and five had AD. All were also scanned for plaques and tangles using NAV4694, aka AZD4694, and MK-6240, respectively.
In his AD/PD presentation, Villemagne showed that 18F-SMBT-1 rapidly enters the brain and most of it quickly clears, resulting in high-contrast images of retained compound (see image above). The tracer reached steady-state levels in the brain within about 50 minutes, as judged by standard uptake value ratios using white matter as the reference region. Its regional distribution in the brain coincided with known expression levels of MAO-B, i.e., the thalamus and caudate retained the greatest amount, and cerebellum and white matter the least. Older, healthy, amyloid-negative volunteers retained twice as much SMBT-1 as did young controls, in keeping with increasing expression of MAO-B in the brain with age.
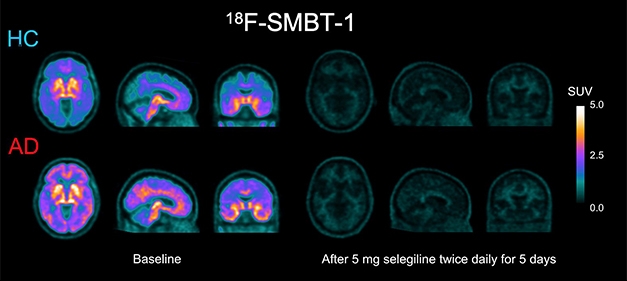
Now You See it, Now You Don’t. Retention of SMBT-1 in the brain of a person with AD and in an age-matched control (left) is all but wiped out by selegiline, an irreversible MAO-B inhibitor (right). The drug quenched up to 85 percent of the signal. [Courtesy of Victor Villemagne.]
Selegeline, the L-enantiomer of deprenyl, cut down the 18F-SMBT-1 signal by 80 to 85 percent, depending on brain region. Because selegeline competes with 18F-SMBT-1 for binding to MAO-B, this reduction is another indication that 18F-SMBT-1’s binding to MAO-B is quite specific. This displacement also suggests that SMBT-1 binds to, or near, the MAO-B catalytic site since deprenyl binds there. Selegeline is approved for treatment of major depression and for people with Parkinson’s disease, in whom it can reduce the need for L-dopa.
What does SMBT-1 reveal about astrogliosis? In this small AIBL cohort, people with AD retained more of this tracer in the posterior cingulate, the supramarginal gyrus, lateral occipital cortex, primary visual cortex, and the angular gyrus, and less in regions such as the hippocampus, parahippocampus, caudate, globus pallidus, and pons. “We found that the close relationship between Aβ deposition and astrogliosis only occurs in some regions of the brain, but not in other regions characterized by high Aβ deposition, such as the ventral frontal cortex and anterior cingulate gyrus,” said Villemagne. “This suggests a more complex and regional relationship than just a 'global ’ [astrocyte] response.” It might be that SMBT-1 PET reflects the progression of disease pathology. Some of the areas that had highest tracer retention, such as the supramarginal gyrus and posterior cingulate, accumulate amyloid early in the disease, Villemagne said.
Next, Villemagne compared SMBT-1 retention in amyloid-positive versus -negative volunteers. The same pattern held; however, additional regions showed higher retention in the amyloid-positive people, including regions of the temporal cortex and the dorsolateral prefrontal cortex. “The most important finding is that those [cognitively normal] controls with higher amyloid in the brain have significantly higher SMBT-1 retention in regions such as the orbitofrontal, posterior cingulate, supramarginal gyrus, temporal occipital regions, and in lateral temporal cortex,” said Villemagne. This suggests that, like deprenyl, the new tracer picks up astrogliosis early in the disease process, albeit with better performance. SMBT-1 has the added advantage of having the 18F isotope for tracking rather than the 11C that labels deprenyl. The short half-life of 11C, circa 20 minutes, limits its use to imaging centers that can create it on-site.
Researchers led by Agneta Nordberg at Stockholm’s Karolinska Institute had previously reported that in autosomal-dominant AD, astrocytosis flares up at the same time as plaques, but not necessarily in the same place (Schöll et al., 2015). This led the scientists to suggest that astrocytosis might even precede plaques. To Villemagne’s mind, astrogliosis does not start a pathological process, but responds to some process going on in the brain. Whether that might be oligomers of Aβ, or some other inflammatory molecule, remains to be seen. "Although I tend to agree with Professor Nordberg, one problem is that whenever the field does not really know what is going on, the default position is to blame ‘the oligomers,’” Villemagne quipped.
SMBT-1 correlated strongly with amyloid, only weakly with tau, and not at all with cognition. “This tells us that Aβ is much better at setting off astrogliosis than is tau,” Villemagne said. Even so, he plans to use the new tracer to study astrogliosis in other diseases, such as ARTAG and LATE, which some believe to be driven primarily by non-Aβ pathologies, such as tau tangles and aggregates of TDP-43, respectively (Kovacs et al., 2016; May 2019 news).
Plasma Portends Astrogliosis
Could GFAP, that oldest of astrocyte markers, hand researchers a plasma surrogate for astrocytosis in Alzheimer’s disease, as well? Only astrocytes seem to produce this structural filament protein in the central nervous system and, like other protein markers such as Aβ, phospho-tau, and NfL, GFAP wends its way into the cerebrospinal fluid and even into the blood stream. Benedet correlated plasma and CSF GFAP with amyloid and tau PET as measured by the same tracers that Villemagne used, namely NAV4694 and MK6240. Benedet, a postdoc in the labs of Kaj Blennow and Henrik Zetterberg at U Gothenburg, used data from 171 volunteers in the TRIAD biomarker study run by Pedro Rosa-Neto at McGill University in Montreal.
In her ADPD talk, Benedet reported that cognitively unimpaired, amyloid-negative older volunteers had more GFAP in their blood than young, healthy controls, showing it rises somewhat with age alone. Amyloid-positive but cognitively healthy older adults had even more. Those with mild cognitive impairment and a positive amyloid test had higher levels still, and people with AD had the highest—about double the cognitively unimpaired Aβ-negative group. The same pattern was seen in CSF, but the differences between groups were neither as stark, nor statistically significant.
Nonetheless, both plasma and CSF GFAP significantly correlated with amyloid as a continuous variable based on global retention of NAV4694 in the brain. Once again, the plasma marker performed best. Drilling down into the data, Benedet found that CSF levels seem to plateau as amyloid accumulates, while plasma GFAP continues to climb. “We are not sure what might explain this pattern,” said Benedet.
For his part, Martins was surprised but found the pattern intriguing. “To my knowledge, no other marker behaves this way,” he told Alzforum. One possibility is that GFAP more easily leaks through the blood-brain barrier than other markers, but to Martins this makes little sense. He suggested testing CSF collected in the AIBL cohort, saying “We need other studies to confirm this pattern.” Still, that Hansson’s group sees plasma GFAP correlating more tightly with brain amyloid than CSF GFAP strengthens the idea that this marker somehow behaves differently than other markers that leave the brain and enter the body’s fluids.
Despite these nuances, plasma GFAP looks promising. In this TRIAD sample, it predicted amyloid positivity with an AUC, i.e., accuracy, of 0.83. It performed slightly better than plasma p-tau181, which posted an AUC of 0.81. CSF GFAP predicted brain amyloid with an AUC of 0.75.
Martins, and also Teunissen, reported similarly tight correlations of plasma GFAP with brain amyloid in their recent papers. “I was surprised how well plasma GFAP performs compared to p-tau,” said Martins. “It is on par with both p-tau181 and p-tau231.”
Martins and colleagues measured plasma GFAP in 100 healthy older people who enrolled in the Kerr Anglican Retirement Village Initiative in Ageing Health, located around Perth, Western Australia. Called KARVIAH, the cohort excludes people with cognitive impairment, though some participants did express concern about their memories, i.e., fell into the category of subjective cognitive complaint. Their average age was 78. As judged by 18F-florbetaben retention in the brain, 33 of them tested positive for amyloid.
They had significantly more GFAP in their plasma than the amyloid-free participants. First author Pratishtha Chatterjee and colleagues found that plasma GFAP predicted brain amyloid with an AUC of 0.80, slightly better than the 0.78 derived from a basic model based on age, sex, and APOE genotype, and better than the AUC of 0.67 for the plasma Aβ42/40 ratio (Chatterjee et al., 2021).
The best prediction of brain amyloid, AUC 0.92, came from combining the basic model with plasma GFAP and Aβ42/40, but this was only marginally better than the basic model plus GFAP alone (AUC 0.91).
Martins collaborates with the Zetterberg/Blennow group in Gothenburg and with Teunissen’s in Amsterdam. He told Alzforum that both had measured GFAP in the same sample and got the same results. All are using a Simoa-based assay from Quanterix. “The assay is very robust,” said Martins. “The coefficient of variation is so low that we only need to measure in singlicate. This makes it cost-effective.”
Teunissen’s group was the first to detect an uptick in GFAP in AD plasma. They measured the marker in 252 people in the Amsterdam Dementia Cohort. Of these, 70 voiced subjective cognitive complaints, 50 had mild cognitive impairment, and 132 had been diagnosed with dementia. All had been scanned for brain amyloid by PET using one of the four commonly used tracers. Again, co-first authors Inge Verberk, Elisabeth Thijssen, and colleagues found that the plasma GFAP concentration, on average, was significantly higher in the 176 people whose amyloid scans were positive than in the 76 amyloid-negative folks. Even among the 70 volunteers with subjective cognitive decline, GFAP was higher among amyloid-positives, suggesting the plasma marker ticks up early in the disease process (Verberk et al., 2020).
In this population, plasma GFAP best predicted amyloid positivity, with an AUC of 0.81, compared to AUCs of 0.73 and 0.71 for plasma Aβ42/40 and plasma NfL, respectively. Among the participants without dementia, the predictive values of all three markers was lower. But even in them, GFAP, with an AUC of 0.76, outperformed both Aβ42/40 and NfL, which had AUCs of 0.67 and 0.63, respectively. Of the three markers, GFAP most strongly associated with global cognitive decline and with decline on specific cognitive domains, including memory, language, attention, and executive function.
In this cohort, too, Teunissen found the best panel for predicting brain amyloid to comprise age, APOE genotype, plasma Aβ42/40, and plasma GFAP. This model returned an AUC of 0.88.
“The field is definitely headed toward algorithms based on multiple markers and risk factors,” said Martins. “I don’t think we yet have a biomarker that can stand alone. We need a good combination to get us across the finish line.”—Tom Fagan
References
News Citations
- Astrocyte Imaging Supports Early Inflammation in the AD Brain
- Introducing LATE—A Common TDP-43 Proteinopathy that Strikes After 80
Paper Citations
- Schöll M, Carter SF, Westman E, Rodriguez-Vieitez E, Almkvist O, Thordardottir S, Wall A, Graff C, Långström B, Nordberg A. Early astrocytosis in autosomal dominant Alzheimer's disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. 2015 Nov 10;5:16404. PubMed.
- Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ, Crary JF, Duyckaerts C, Ghetti B, Halliday GM, Ironside JW, Love S, Mackenzie IR, Munoz DG, Murray ME, Nelson PT, Takahashi H, Trojanowski JQ, Ansorge O, Arzberger T, Baborie A, Beach TG, Bieniek KF, Bigio EH, Bodi I, Dugger BN, Feany M, Gelpi E, Gentleman SM, Giaccone G, Hatanpaa KJ, Heale R, Hof PR, Hofer M, Hortobágyi T, Jellinger K, Jicha GA, Ince P, Kofler J, Kövari E, Kril JJ, Mann DM, Matej R, McKee AC, McLean C, Milenkovic I, Montine TJ, Murayama S, Lee EB, Rahimi J, Rodriguez RD, Rozemüller A, Schneider JA, Schultz C, Seeley W, Seilhean D, Smith C, Tagliavini F, Takao M, Thal DR, Toledo JB, Tolnay M, Troncoso JC, Vinters HV, Weis S, Wharton SB, White CL 3rd, Wisniewski T, Woulfe JM, Yamada M, Dickson DW. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016 Jan;131(1):87-102. Epub 2015 Dec 10 PubMed.
- Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, Verberk IM, Dave P, Taddei K, Sohrabi HR, Zetterberg H, Blennow K, Teunissen CE, Vanderstichele HM, Martins RN. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021 Jan 11;11(1):27. PubMed.
- Verberk IM, Thijssen E, Koelewijn J, Mauroo K, Vanbrabant J, de Wilde A, Zwan MD, Verfaillie SC, Ossenkoppele R, Barkhof F, van Berckel BN, Scheltens P, van der Flier WM, Stoops E, Vanderstichele HM, Teunissen CE. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020 Sep 28;12(1):118. PubMed.
Other Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.