What If It’s Not Garden-Variety AD? Telling Variants Apart by Where Tau Is
Quick Links
Scientists are increasingly confident that tau PET by and large matches up with expected regions and symptoms in Alzheimer’s disease, but does this hold true for diseases beyond typical AD, as well? This is an even younger area of tau PET exploration, because these cases are rarer, hence fewer patients have been scanned thus far. That said, at the 9th Human Amyloid Imaging conference last month in Miami Beach, Florida, several scientists showed early data hinting that tau PET might be useful here, too. Researchers were particularly excited by the prospect that tau PET might align quite well with other markers of neurodegeneration, particularly FDG PET. If larger studies bear out this trend, they may draw a sharp line between a specific protein pathophysiology and a person’s symptoms.
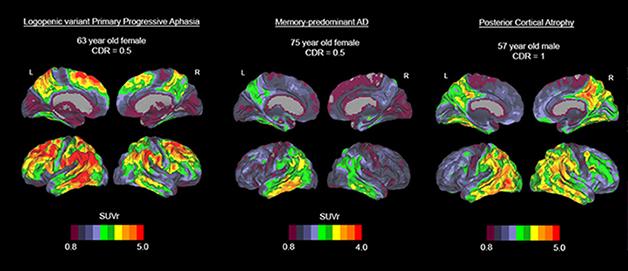
Three cases with distinct variants of Alzheimer’s disease show AV1451 uptake in distinct brain regions. A woman with PPA (left) shows an intense signal in language areas while her frontal and occipital areas are spared; a woman with mild typical AD (middle) appears to have tau pathology in the inferior temporal cortex; a man with posterior cortical atrophy (right) shows the strong signal in occipital and tempoparietal regions. [Image courtesy of Rik Ossenkoppele and Gil Rabinovici.]
At HAI, Eli Lilly’s Adam Schwarz told the audience that T807/AF1451 might be able to pick up two known variants of Alzheimer’s disease. One involves a hippocampal-sparing pattern, marked by fewer neurofibrillary tangles in the hippocampus and amygdala than typical AD, the other a so-called limbic-predominant variant marked by more tangles in the same areas. Concurring with this, Gil Rabinovici of the University of California, San Francisco, who won the 2015 Christopher Clark Award at this year's meeting, noted findings in his group that the regional pattern of tau, but not amyloid, PET tends to jibe with the regions thought to underlie the particular clinical symptoms in patients with atypical forms of AD.
As an example of Rabinovici’s group’s findings, Rik Ossenkoppele showed a poster about five patients with an AD variant called posterior cortical atrophy. People with PCA have visual and certain cognitive symptoms, but their memory initially stays intact. They have atrophy in the back areas of their brain, and Alzheimer’s pathology (see Jul 2012 conference news). Ossenkoppele and colleagues showed tau pathology in the expected occipitotemporal and –parietal regions in the back of the brain. Strikingly, in the PCA cases, and a handful of other AD-variant cases, the researchers saw virtual mirror images in the uptake of T807/AV1451 and 18F FDG, which measures neural activity. In other words, brain metabolism was abnormally low where tau pathology was high. This tight regional relationship was absent with amyloid PET, which is distributed more broadly across the association neocortex. Ossenkoppele published one PCA case report this month (Ossenkoppele et al., 2015).

Voxel-wise comparisons between 16 controls and four patients with posterior cortical atrophy, a visual variant of Alzheimer’s disease, using 18F AV1451 PET (tau pathology, green), 18F FDG PET (glucose metabolism, red), and 11C PIB (amyloid deposition, blue). This figure demonstrates that tau aggregations strikingly mirror the posterior hypometabolic pattern on FDG-PET, while amyloid deposition is present in both clinically affected posterior regions and less-affected frontal areas. [Image courtesy of Rik Ossenkoppele and Gil Rabinovici.]
Tau PET carries much hope of enabling a molecular diagnosis in frontotemporal dementia. With this complex set of diseases, it is difficult for a clinician to predict which particular protein pathophysiology likely underlies a given patient’s clinical syndrome. Doing that, however, is important not only to managing the patient’s disease, but also for evaluating protein-specific therapies in clinical trials. Some FTD variants, for example progressive nuclear palsy (PSP), almost always have tau pathology. On the other hand, with behavioral variant FTD, the most common form, it’s about a 50-50 tossup between tau and TDP-43, and yet other FTDs fall in between. “Tau PET could really be a game-changer here,” Rabinovici said.

Voxelwise comparison of AV1451 retention in five people with progressive supranuclear palsy versus normal controls shows increased signal in globus pallidus, substantia nigra and subthalamic nucleus in this tau disease. [Image courtesy of Gil Rabinovici and Bill Jagust.]
At HAI, Rabinovici showed how T807/AV1451 performed for the first 13 non-AD cases scanned at UCSF. For example, the tau scan of a PSP case with a full deck of symptoms largely corresponded to the brain regions one would expect based on the published neuropathological staging system for the disease (Williams et al., 2007). However, the tracer also bound some of the same regions in cognitively normal controls. In a group of five PSP cases and 16 controls, the suspected PSP regions—substantia nigra, globus pallidus, and subthalamic nucleus—did have more binding in patients, but individual cases overlapped with controls. This could limit T807/AV1451’s ability to pick up early stages of PSP, Rabinovici said.
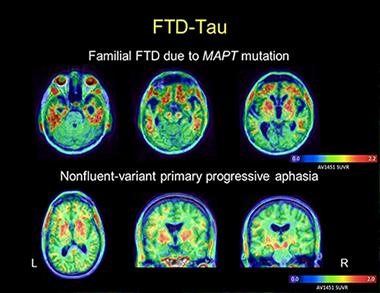
AV1451 images in a patient with behavioral-variant FTD due to MAPT mutation reveal tracer uptake in the frontal and anterior temporal lobes (top row). Images in a patient with nonfluent PPA show more asymmetric uptake in the left than the right peri-Sylvian cortex, a part of the brain’s language networks (bottom row). In both cases, the patterns of tracer uptake conform to the expected distribution of tau pathology as described in postmortem studies. [Image courtesy of Gil Rabinovici and Bill Jagust]
Of Rabinovici’s two primary progressive aphasia patients who have been scanned, one with the nonfluent variant (which is usually a tau disease) showed a plausible AV1451 distribution based on postmortem pathology of this syndrome. However, a person with semantic variant PPA had significant uptake, too, even though this disease is usually tau negative at postmortem. Likewise, for two bvFTD cases, one person’s tau scan instantly made sense and one did not. A case with a pathogenic tau mutation had a “spectacular” scan with intensive uptake in only the predicted regions, said Rabinovici. The other case was puzzling because the person carried a C9ORF72 repeat expansion and therefore would have been expected to have no T807/AV1451 uptake. Yet there it was—in degenerating white-matter tracts.
Similarly, of two suspected cerebral traumatic encephalopathy (CTE) cases, one showed T807/AV1451 uptake in regions matching neuropathology reported previously by Ann McKee of Boston University, and the other did not.
Brad Dickerson, who runs an FTD clinic at Massachusetts General Hospital, is finding much the same thing in that some scans fit the picture, some do not. At HAI, Dickerson, working with Stephen Gomperts and others, showed expected T807/AV1451 uptake in the basal ganglia, brainstem, and other predicted regions in PSP. Dickerson saw expected, asymmetric uptake in the dorsolateral prefrontal and insular cortex in nonfluent variant PPA, but also unexpected uptake in anterior temporal cortex in semantic variant PPA. Five tau mutation carriers, some asymptomatic and some with symptoms of bvFTD, retained T807/AV1451 in their frontal, insular, and anterior temporal cortices. In all cases, uptake and atrophy occurred in the same regions, Dickerson said.
For neuropathology correlations and technical research on the tracer’s behavior, see Part 5 of this series.—Gabrielle Strobel
References
News Citations
- Researchers Join to Draw Posterior Cortical Atrophy Out of Shadows
- Mixed Bag on Tau Tracer Validation, Kinetics: Some Things Fit, Some Don’t
Paper Citations
- Ossenkoppele R, Schonhaut DR, Baker SL, O'Neil JP, Janabi M, Ghosh PM, Santos M, Miller ZA, Bettcher BM, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol. 2015 Feb;77(2):338-42. Epub 2014 Dec 17 PubMed.
- Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson's syndrome. Brain. 2007 Jun;130(Pt 6):1566-76. PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
University of Arkansas for Medical Sciences
It seems the following papers would be relevant to the C9ORF72 data: Bieniek et al., 2013; King et al., 2013.
References:
Bieniek KF, Murray ME, Rutherford NJ, Castanedes-Casey M, Dejesus-Hernandez M, Liesinger AM, Baker MC, Boylan KB, Rademakers R, Dickson DW. Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol. 2012 Sep 28; PubMed.
King A, Al-Sarraj S, Troakes C, Smith BN, Maekawa S, Iovino M, Spillantini MG, Shaw CE. Mixed tau, TDP-43 and p62 pathology in FTLD associated with a C9ORF72 repeat expansion and p.Ala239Thr MAPT (tau) variant. Acta Neuropathol. 2012 Sep 28; PubMed.
Make a Comment
To make a comment you must login or register.