Does “Good” Cholesterol Increase Your Risk of Alzheimer’s?
Quick Links
What is good for the heart is good for the brain, right? A new Mendelian randomization study of 440,000 people complicates the picture. In the May 17 JAMA Network Open, scientists led by Ruth Frikke-Schmidt, Copenhagen University Hospital–Rigshospitalet, Denmark, reported that not only does high systolic blood pressure (SBP) increase a person’s risk of getting Alzheimer’s disease—no surprise there—but so, apparently, does high plasma HDL cholesterol. HDL? While the blood pressure association confirms epidemiological priors, high HDL had been thought to lower risk of both cardiovascular disease and amyloid pathology. It's important to learn what gives, because knowing which modifiable risk factors cause AD can guide lifestyle interventions and deepen scientists' understanding of disease pathogenesis.
- Twelve modifiable risk factors for Alzheimer's disease were tested by Mendelian randomization.
- High blood pressure and high HDL levels appear to worsen the odds.
- The latter may provide insights into AD pathogenesis.
Others were puzzled by the HDL association. “The paradoxical association between high HDL-C, the so called ‘good cholesterol,’ and AD risk needs more discussion,” wrote Ling Li, University of Minnesota, Minneapolis.
Scientists believe 40 percent of dementia cases can be prevented because they are caused by modifiable risk factors, such as obesity, smoking, and lack of exercise (Aug 2020 conference news; Jul 2022 news). However, teasing apart which ones cause the disease using observational studies is tricky. Reverse causality, where early stage disease changes the risk factor rather than the other way around, and confounds, such as socioeconomic and lifestyle factors, can obscure true links.
To avoid these pitfalls, first author Jiao Luo and colleagues turned to Mendelian randomization, an analysis method that correlates disease outcomes with genetic variants that associate with specific traits, such as SBP, and act as proxies for lifelong exposure to them (Oct 2018 news; May 2017 news).
The researchers tied 12 known modifiable risk factors to as few as 100 to as many as several thousand single-nucleotide variants from six previous genome-wide association studies, generating polygenic risk scores for each factor. They included plasma levels of six lipid molecules: LDL cholesterol, HDL cholesterol, triglycerides, ApoA1, and ApoB. They also created risk scores for systolic blood pressure, diastolic blood pressure, body mass index, Type 2 diabetes, smoking status, alcohol consumption, and years of education. This collaboration, among almost 100 scientists, calculated those polygenic scores for 39,100 adults, ages 72 to 83, who had been clinically diagnosed with AD, and 401,600 healthy people, aged 51 to 80. All came from the European Alzheimer’s & Dementia Biobank.
Of the 12 modifiable factors, only polygenic scores for SBP, HDL, and years of education associated with AD in this large sample. Each 10-mmHg increase in blood pressure, or one standard deviation rise in HDL, corresponded to a 22 or 10 percent jump in risk, respectively (see image below). “A 22 percent increased risk is substantial on a population level,” Frikke-Schmidt told Alzforum.
People carrying variants linked to completing more years of education had lower odds of developing AD, in line with previous epidemiological studies. For the other nine risk factors, MR analysis was inconclusive or suggested no association.
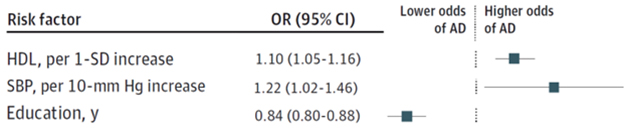
AD Risk Factors. Mendelian randomization suggests that high HDL cholesterol or blood pressure increases a person’s odds of developing AD, while attending school for longer decreases risk. [Courtesy of Luo et al., JAMA Network Open, 2023.]
What is the upshot? For the SBP association, the authors concluded that maintaining a healthy blood pressure can reduce risk of dementia. This extends to people whose high SBP is due to diet or lifestyle. “No matter why you have high blood pressure or HDL, it’s a cause of AD that should be treated or avoided,” said Frikke-Schmidt.
What to make of high HDL is more complicated. Rather than suggesting that people lower their HDL, Frikke-Schmidt thinks the cholesterol finding should encourage research on how high levels of the lipid contribute to dementia risk. Others agreed. “[HDL cholesterol level] does not reflect the complex and dynamic composition, structure, and function of [the lipid],” wrote Li.
Cheryl Wellington of the University of British Columbia, Canada, noted that scientists are moving toward measuring aspects of HDL’s function, such as cholesterol efflux activity, where the lipoprotein carts cholesterol out of cells and into the blood stream. “These are likely to be important in understanding how HDL effects Alzheimer’s disease risk,” Wellington wrote.
How might high HDL drive AD risk? The authors think it disrupts lipid particle homeostasis in the brain. In the cerebrospinal fluid, Apolipoprotein E transfers cholesterol to Apolipoprotein A1 in small HDL particles, which carry the lipids to the blood (Koch et al., 2001; Hubin et al., 2019). This clearance also helps remove Aβ from the brain as the peptide comes along with ApoE and attaches to HDL particles (Van Valkenburgh et al., 2021). However, high levels of HDL drive formation of large lipid particles, which the authors think are less efficient at transporting lipids and clearing Aβ.
Indeed, the concentration in the CSF of small, but not large, HDL particles correlated with reduced cerebral amyloid load and better cognitive performance (Martinez et al., 2022). “Understanding the mechanisms that promote the formation of small HDL particles in the brain holds promise for research aimed at reducing the risk of AD,” wrote Hussein Yassine, University of Southern California, Los Angeles (comment below).
One concern is that this massive study incorporated no diagnostic biomarker analysis to classify dementia cases as being indeed AD. “The associations observed here cannot be attributed with any certainty to plaque and tangle disease,” noted David Knopman at the Mayo Clinic in Rochester, Minnesota.—Chelsea Weidman Burke
References
News Citations
- Lancet Commission’s Dementia Hit List Adds Alcohol, Pollution, TBI
- In the U.S., 40 Percent of All-Cause Dementia Is Preventable
- Intelligence Matters More for Brain Reserve, but Education Helps
- No, Being Thin Does Not Lead to Alzheimer’s Disease
Paper Citations
- Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001 Jul;42(7):1143-51. PubMed.
- Hubin E, Verghese PB, van Nuland N, Broersen K. Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation. FEBS Lett. 2019 Jun;593(11):1144-1153. Epub 2019 May 27 PubMed.
- Van Valkenburgh J, Meuret C, Martinez AE, Kodancha V, Solomon V, Chen K, Yassine HN. Understanding the Exchange of Systemic HDL Particles Into the Brain and Vascular Cells Has Diagnostic and Therapeutic Implications for Neurodegenerative Diseases. Front Physiol. 2021;12:700847. Epub 2021 Sep 6 PubMed.
- Martinez AE, Weissberger G, Kuklenyik Z, He X, Meuret C, Parekh T, Rees JC, Parks BA, Gardner MS, King SM, Collier TS, Harrington MG, Sweeney MD, Wang X, Zlokovic BV, Joe E, Nation DA, Schneider LS, Chui HC, Barr JR, Han SD, Krauss RM, Yassine HN. The small HDL particle hypothesis of Alzheimer's disease. Alzheimers Dement. 2022 Apr 13; PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- European Alzheimer’s & Dementia Biobank Mendelian Randomization (EADB-MR) Collaboration, Luo J, Thomassen JQ, Bellenguez C, Grenier-Boley B, de Rojas I, Castillo A, Parveen K, Küçükali F, Nicolas A, Peters O, Schneider A, Dichgans M, Rujescu D, Scherbaum N, Jürgen D, Riedel-Heller S, Hausner L, Porcel LM, Düzel E, Grimmer T, Wiltfang J, Heilmann-Heimbach S, Moebus S, Tegos T, Scarmeas N, Clarimon J, Moreno F, Pérez-Tur J, Bullido MJ, Pastor P, Sánchez-Valle R, Álvarez V, Boada M, García-González P, Puerta R, Mir P, Real LM, Piñol-Ripoll G, García-Alberca JM, Royo JL, Rodriguez-Rodriguez E, Soininen H, Kuulasmaa T, de Mendonça A, Mehrabian S, Hort J, Vyhnalek M, van der Lee S, Graff C, Papenberg G, Giedraitis V, Boland A, Bacq-Daian D, Deleuze JF, Nicolas G, Dufouil C, Pasquier F, Hanon O, Debette S, Grünblatt E, Popp J, Benussi L, Galimberti D, Arosio B, Mecocci P, Solfrizzi V, Parnetti L, Squassina A, Tremolizzo L, Borroni B, Nacmias B, Sorbi S, Caffarra P, Seripa D, Rainero I, Daniele A, Masullo C, Spalletta G, Williams J, Amouyel P, Jessen F, Kehoe P, Magda T, Rossi G, Sánchez-Juan P, Sleegers K, Ingelsson M, Andreassen OA, Hiltunen M, Van Duijn C, Sims R, van der Flier W, Ruiz A, Ramirez A, Lambert JC, Frikke-Schmidt R. Genetic Associations Between Modifiable Risk Factors and Alzheimer Disease. JAMA Netw Open. 2023 May 1;6(5):e2313734. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Minnesota, Twin Cities
This comprehensive genetic study shows that high HDL-cholesterol (HDL-C) concentrations and high systolic blood pressure (SBP) are associated with greater risk of AD. While the detrimental effects of high SBP are well-documented, the paradoxical association between high HDL-C, the so called “good cholesterol,” and AD risk needs more discussion.
First, although HDL-C has been widely used as a maker for HDL, it does not reflect the complex and dynamic composition, structure, and function of HDL. It has long been recognized that HDL can serve as a double-edged sword, playing both anti-inflammatory and pro-inflammatory roles in cardiovascular disease (Navab et al., 2005). In fact, recent studies have demonstrated a U-shaped relationship between HDL-C and adverse outcomes, with both low and extremely high HDL increasing the risk (Madsen et al., 2017; Huang et al., 2020).
Second, although HDL-C concentrations were determined by SNPs in well-known genes, most of those associations were based on only a single time-point measurement, as indicated in the article. The function of HDL can be modified by many lifestyle and environmental factors, independent of genetically determined HDL-C concentrations. It is also unclear whether the effects of any potential epigenetic and/or post-translational modifications were taken into consideration. Further, Richardson et al. reported that almost half of the SNPs were associated with more than one lipid-related trait (Richardson et al., 2020). Although the results on HDL-C were adjusted for LDL and triglycerides in this article, other components of lipoproteins might be involved, due to the complexity and dynamic nature of lipoprotein metabolism.
Third, as high HDL-C generally correlates inversely with the incidence of cardiovascular disease, the association of high HDL-C with AD could be the result of better cardiovascular health and longevity. The fact that the participants with AD were clearly older (72-83 years) than controls (51-80 years) supports this possibility.
Finally, the impact of HDL on AD-related processes has been investigated experimentally by genetic manipulation of APOA-I expression. Elevating HDL-C or enhancing HDL function by APOA-I overexpression rescued cognitive function and attenuated neuroinflammation and cerebral amyloid angiopathy (CAA) in AD mice, whereas APOA-I deficiency exacerbated cognitive deficits and CAA in those same mice (Lewis et al., 2010; Lefterov et al., 2010; Oct 2010 news). Notably, while genetic manipulation of apoA-I expression did not affect parenchymal amyloid pathology in earlier studies (Fagan et al., 2004; Lefterov et al., 2010; Lewis et al., 2010), a later study showed that APOA-I deficiency worsened both parenchymal and vascular amyloid deposition in AD mice (Button et al., 2019). Overall, these findings support the benefits of HDL in AD and the widely held belief that “what is good for the heart is also good for the brain.”
Based on these discussions, the association between high HDL-C and AD risk should be interpreted with caution. In the meantime, these findings underscore the role of HDL in AD and the need for further investigation in this direction.
References:
Button EB, Boyce GK, Wilkinson A, Stukas S, Hayat A, Fan J, Wadsworth BJ, Robert J, Martens KM, Wellington CL. ApoA-I deficiency increases cortical amyloid deposition, cerebral amyloid angiopathy, cortical and hippocampal astrogliosis, and amyloid-associated astrocyte reactivity in APP/PS1 mice. Alzheimers Res Ther. 2019 May 13;11(1):44. PubMed.
Fagan AM, Christopher E, Taylor JW, Parsadanian M, Spinner M, Watson M, Fryer JD, Wahrle S, Bales KR, Paul SM, Holtzman DM. ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis. Am J Pathol. 2004 Oct;165(4):1413-22. PubMed.
Huang YQ, Liu XC, Lo K, Liu L, Yu YL, Chen CL, Huang JY, Feng YQ, Zhang B. The U Shaped Relationship Between High-Density Lipoprotein Cholesterol and All-Cause or Cause-Specific Mortality in Adult Population. Clin Interv Aging. 2020;15:1883-1896. Epub 2020 Oct 2 PubMed.
Lefterov I, Fitz NF, Cronican AA, Fogg A, Lefterov P, Kodali R, Wetzel R, Koldamova R. Apolipoprotein A-I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. J Biol Chem. 2010 Nov 19;285(47):36945-57. Epub 2010 Aug 25 PubMed.
Lewis TL, Cao D, Lu H, Mans RA, Su YR, Jungbauer L, Linton MF, Fazio S, LaDu MJ, Li L. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J Biol Chem. 2010 Nov 19;285(47):36958-68. Epub 2010 Sep 16 PubMed.
Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017 Aug 21;38(32):2478-2486. PubMed.
Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, Hough G, Bachini E, Grijalva VR, Wagner AC, Shaposhnik Z, Fogelman AM. The double jeopardy of HDL. Ann Med. 2005;37(3):173-8. PubMed.
Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, Holmes MV. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020 Mar;17(3):e1003062. Epub 2020 Mar 23 PubMed.
University of Southern California
The European Alzheimer’s & Dementia Biobank Collaboration employed a mendelian randomization (MR) design to investigate whether single-nucleotide variants in 39,106 participants diagnosed with Alzheimer's disease and 401,577 control participants without AD can identify modifiable risk factors for AD that could serve as intervention targets. The basis of this MR approach lies in the notion that genetic variants randomly assigned at conception help mitigate confounding factors and reverse causation, which are two major limitations of observational studies that often lead to confusion between association and causation.
The study reveals two associations: higher risk of AD being linked to elevated levels of HDL cholesterol, and increased systolic blood pressure. While the connection between higher systolic blood pressure and greater AD risk is expected, the association between high HDL cholesterol and increased risk might seem surprising. Traditionally, HDL or "good cholesterol," has been regarded as a protective cardiovascular risk factor. However, extensive research in HDL biology over the past two decades has illustrated its complexity and explained why targeting HDL cholesterol is not effective. Unlike LDL, HDL particles exhibit significant variations in size, shape, composition, and function. Moreover, HDL cholesterol does not adequately represent HDL function. HDL particles in the bloodstream predominantly consist of ApoA-I particles, whereas in the brain, they are composed of ApoE (Van Valkenburgh et al., 2021). Notably, genetic variations in ApoA-I did not show any association with the risk of AD in this cohort.
The initial step in HDL formation involves the interaction of ApoA-I in plasma or ApoE in the brain with ATP-binding cassette 1 (ABCA-1), resulting in the production of small HDL particles that are not yet fully loaded with cholesterol but exhibit high cholesterol efflux activity. These small HDL particles then undergo remodeling through the actions of LCAT, PLTP, CETP, ABCG1, and SR-BI to acquire more cholesterol and form larger, cholesterol-rich particles. Elevated HDL cholesterol levels sometimes may indicate reduced reverse cholesterol transport, as observed in the case of loss-of-function mutations in the SR-BI receptor responsible for HDL liver uptake and cholesterol clearance (Rigotti et al., 1997). Conversely, loss-of-function mutations in ABCA1, responsible for generating small HDL particles, are associated with greater AD risk (Nordestgaard et al., 2015). Small HDL particle concentrations are not captured by measuring HDL cholesterol levels, and they correlate with better measures of cognitive function performance and lower cerebral amyloid accumulation (Martinez et al., 2022).
Although it remains unclear whether small HDL particles formed in the periphery can cross into the brain, unlike large HDL particles their concentrations in the bloodstream and the brain are correlated (Martinez et al., 2022).
The findings of this study prompt us to question the value of HDL cholesterol and whether reducing HDL cholesterol levels can translate into a lower AD risk. Recent advancements in HDL biology suggest a shift from thinking about HDL cholesterol to focusing on HDL particle numbers and HDL's cholesterol efflux activity. Understanding the mechanisms that promote the formation of small HDL particles in the brain holds promise for research aimed at reducing the risk of AD.
References:
Van Valkenburgh J, Meuret C, Martinez AE, Kodancha V, Solomon V, Chen K, Yassine HN. Understanding the Exchange of Systemic HDL Particles Into the Brain and Vascular Cells Has Diagnostic and Therapeutic Implications for Neurodegenerative Diseases. Front Physiol. 2021;12:700847. Epub 2021 Sep 6 PubMed.
Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12610-5. PubMed.
Nordestgaard LT, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Loss-of-function mutation in ABCA1 and risk of Alzheimer's disease and cerebrovascular disease. Alzheimers Dement. 2015 Jun 13; PubMed.
Martinez AE, Weissberger G, Kuklenyik Z, He X, Meuret C, Parekh T, Rees JC, Parks BA, Gardner MS, King SM, Collier TS, Harrington MG, Sweeney MD, Wang X, Zlokovic BV, Joe E, Nation DA, Schneider LS, Chui HC, Barr JR, Han SD, Krauss RM, Yassine HN. The small HDL particle hypothesis of Alzheimer's disease. Alzheimers Dement. 2022 Apr 13; PubMed.
It is great to see this study, which used the largest genomic consortia to date to systematically investigate the causal relationship between modifiable risk factors and Alzheimer’s disease. An additional strength of the study is that it highlighted the potential influence of proxy AD cases when evaluating causal relationships between behavioral risk factors and AD. Findings from this study will inspire the exploration of novel intervention and prevention avenues.
In addition to established associations between modifiable risk factors and AD (e.g., educational attainment), the authors reported that genetically determined high HDL cholesterol and high systolic blood pressure (SBP) were associated with higher odds of AD. These findings are very interesting. The HDL association, in particular, contrasts previous studies reporting no association, or a protective association, between high HDL cholesterol and AD.
The finding of a positive causal association between SBP and AD is also interesting, particularly as such a relationship is observed only after adjusting for diastolic blood pressure.
As the authors highlight, it is crucial to replicate these findings in non-European populations. Additionally, now that large, well-powered GWAS, such as the EADB are available, it would be interesting to investigate any potential medication effects, causal associations with HDL sub-fractions, or causal, nonlinear blood pressure associations.
Make a Comment
To make a comment you must login or register.