Brain Within a Brain? Nested Organoids a New Way to Study Human Microglia
Quick Links
Could putting a tiny brain inside a larger brain help study disease? As far-fetched as the idea might sound, the approach could allow scientists to investigate human microglia in near-physiological conditions. These cells are notoriously mercurial, changing gene expression to adapt to their environment. Thus, microglia in a dish behave differently to those in a human brain. In the May 11 Cell, researchers led by Fred Gage at the Salk Institute for Biological Studies in La Jolla, California, skirt this problem by growing microglia in human cerebral organoids inside mouse brains. The host brain provides blood flow and nutrients, the nested human organoid the right environmental cues. In this system, microglial gene expression resembled that seen in human brain, the authors found. “In effect, this gives us the ability to peek into the human brain dynamically,” co-first author Simon Schaefer, now at the Technical University of Munich, told Alzforum.
- Microglia in human brain organoids survive long-term when planted into mouse brain.
- The cells assume mature homeostatic states that resemble those in human brain.
- The system can be used to study cell- and patient-specific aspects of disease.
“It is very exciting to see a research paper like this,” said Shauna Yuan at the University of Minnesota, Minneapolis.
“The advantage of this new method is the possibility of examining the interaction of human microglia and neuronal cells in vivo, during development, and in a disease microenvironment.”

Little Brain, Big Brain. Human erythromyeloid, i.e. microglial, precursors (red) are added to cultured cerebral organoids, then transplanted into mouse brain for long-term survival. [Courtesy of Schaefer et al., Cell.]
Previous studies had alerted the field that microglia change state as soon as they are removed from the brain and placed in a dish (Jul 2016 conference news; Jun 2017 news). One way to get around this problem, yet still study human microglia in an accessible system, would be to culture them inside cerebral organoids, pea-sized brains grown from stem cells (Aug 2013 news; Jun 2019 news; Oct 2019 news).

Microglial Colonization. Microglial precursors (bright red) migrate throughout a cultured cerebral organoid (green, left). [Courtesy of Schaefer et al., Cell.]
Gage and colleagues set out to do this. However, cerebral organoids do not give rise to microglia. The immune cells have a distinct developmental origin, forming from erythromyeloid progenitors (EMPs) that migrate into the nascent brain. To replicate this developmental process, joint first authors Schaefer and Abed Mansour, now at the Hebrew University of Jerusalem, generated fluorescently labeled EMPs from human induced pluripotent stem cells, using a protocol developed by Mathew Blurton-Jones and colleagues at the University of California, Irvine (Abud et al., 2017). They cultured these EMPs with separately grown human cortical organoids that were 5 to 6 weeks old, the stage when neurogenesis begins. The EMPs migrated into the organoid within 12 hours, and a week later, 96 percent of them expressed the microglial master regulator PU.1, indicating they had differentiated.
But there was a problem. The microglia maintained an expression profile typical of fetal brain, and over three months in culture, nearly all of them died off. The cerebral organoids were not providing the right cues for their maturation and long-term survival. To get around this, Schaefer and Mansour used a protocol previously established in the Gage lab for transplanting cerebral organoids into the brains of immunocompromised mice (Mansour et al., 2018). As expected, the host brain vascularized the organoid, creating a more sustainable environment.
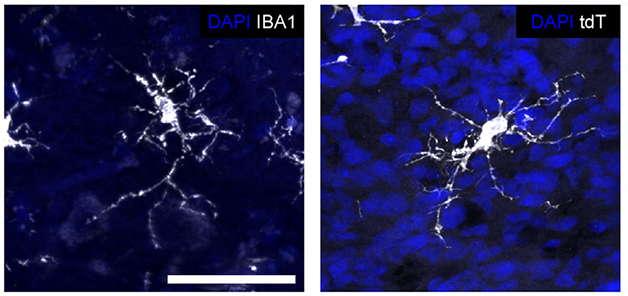
Mature Microglia. Human microglia (white) in a transplanted cerebral organoid (right) assume the same ramified shape as do microglia in human brain (left). Nuclei are blue. [Courtesy of Schaefer et al., Cell.]
In this system, the human microglia thrived. Within eight weeks, nearly all of them expressed mature markers such as TMEM119, P2RY12, and IBA1. They assumed the branching shape of mature microglia, and two-photon time-lapse imaging showed them surveying their environment, as homeostatic microglia do in the brain. The cells even responded to an injury by extending their processes toward the lesion (see movie below). Moreover, human microglia stayed put within the graft, not migrating into the surrounding mouse brain. This may be because the mouse version of the microglial growth factor CSF-1 does not sustain human cells, the authors noted. They found that once transplanted into the mouse brain, the human organoid began producing its own CSF-1.
Emergency Responders. Organoid microglia rapidly react to an injury, extending long cellular processes to the site. [Movie courtesy of Schafer et al., Cell, 2023.]
RNA-Seq studies highlighted the robustness of this model for producing brain-like microglia. Comparing transcriptional profiles of grafted and cultured organoids, only the former contained homeostatic microglia, while in the latter the cells expressed genes for cellular stress and apoptosis. Microglia gene-expression profiles in the graft closely matched those seen in live human brain samples (Geirsdottir et al., 2019; Sankowski et al., 2019).
These in vivo organoids offer an alternative to chimeric mice developed by researchers in the Blurton-Jones and Bart De Strooper labs, in which human microglia are transplanted directly into mouse brain (Aug 2019 news). Gage and colleagues found that directly transplanted human microglia had expression profiles similar to mouse microglia, suggesting the mouse environment influenced the human cells, whereas those in nested organoids did not.
Finally, the authors demonstrated that this approach can be used to study disease. They grew cortical organoids from iPSCs made from people with autism spectrum disorder (ASD), then populated them with EMPs generated from healthy controls. The microglia that emerged looked abnormal, with large cell bodies and too many filopodia, similar to reactive microglia seen in ASD brain (Morgan et al., 2010; Lee et al., 2017). This suggested to the authors that the microglial changes in ASD are not due to microglial gene variants, but to a response to the brain environment.—Madolyn Bowman Rogers
References
News Citations
- When a Microglia Is No Longer a Microglia
- What Makes a Microglia? Tales from the Transcriptome
- Mini Brain in a Dish Models Human Development
- Reproducible Brain Organoids Could Offer New Models for Research
- It Bleeds! New Mini-Brains Sport Functioning Blood Vessels
- Human Microglia Make Themselves at Home in Mouse Brain
Paper Citations
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
- Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018 Jun;36(5):432-441. Epub 2018 Apr 16 PubMed.
- Geirsdottir L, David E, Keren-Shaul H, Weiner A, Bohlen SC, Neuber J, Balic A, Giladi A, Sheban F, Dutertre CA, Pfeifle C, Peri F, Raffo-Romero A, Vizioli J, Matiasek K, Scheiwe C, Meckel S, Mätz-Rensing K, van der Meer F, Thormodsson FR, Stadelmann C, Zilkha N, Kimchi T, Ginhoux F, Ulitsky I, Erny D, Amit I, Prinz M. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell. 2019 Dec 12;179(7):1609-1622.e16. PubMed.
- Sankowski R, Böttcher C, Masuda T, Geirsdottir L, Sagar, Sindram E, Seredenina T, Muhs A, Scheiwe C, Shah MJ, Heiland DH, Schnell O, Grün D, Priller J, Prinz M. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019 Nov 18; PubMed. Correction.
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010 Aug 15;68(4):368-76. PubMed.
- Lee AS, Azmitia EC, Whitaker-Azmitia PM. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav Immun. 2017 May;62:193-202. Epub 2017 Feb 1 PubMed.
Further Reading
Primary Papers
- Schafer ST, Mansour AA, Schlachetzki JC, Pena M, Ghassemzadeh S, Mitchell L, Mar A, Quang D, Stumpf S, Ortiz IS, Lana AJ, Baek C, Zaghal R, Glass CK, Nimmerjahn A, Gage FH. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 2023 May 11;186(10):2111-2126.e20. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UK Dementia Research Institute@UCL and VIB@KuLeuven
VIB-KU Leuven
VIB-KU Leuven
VIB-KU Leuven
Humanized systems are becoming increasingly popular due to their translational potential. However, current in vitro models do not fully recapitulate important features, such as acquisition of mature cell states, three-dimensional structure, or cell-to-cell communication.
The article by Schaefer and colleagues establishes a new paradigm to study human neuron-glia communication by integrating human-derived microglia progenitors into a human brain organoid (HBOs). This is the first time that such a model is described in vivo, and it would help to elucidate the complex interplay between the innate immune system and neurons in neurological disorders, such as Autism or Alzheimer’s disease.
As described previously (Gosselin et al., 2017; Mancuso et al., 2019; Hasselmann et al., 2019; Xu et al., 2020), human-derived microglia produced in vitro lack key environmental cues to fully transition to a mature state, locking them in an artificial activation phenotype. This article also demonstrates that the integration of human microglia into HBOs in vitro is not enough to rescue this phenotype, suggesting that human-derived microglia need multiple brain-derived factors to reach maturation. In vivo, the HBO appears to secrete key human microglia factors—CSF-1 and IL-34—that influence the maturation of human microglia.
This is an improvement compared to current microglia xenotransplantation systems (Mancuso et al., 2019; Hasselmann et al., 2019; Xu et al., 2020), where, to achieve a successful engraftment there is the need to express humanized cytokines, such as CSF1 (Mancuso et al., 2019; Xu et al., 2020) or CSF1/GM-CSF/IL3 (Hasselmann et al., 201). This model also goes one step further to decipher not only the individual contribution from each cell type, but the complex cross talk between them. The presence of human neurons in this experimental model is a very valuable addition, since we have demonstrated that xenotransplantation of human neurons recapitulates key pathological hallmarks of AD when exposed to amyloid pathology (Balusu et al., 2022). However, an in-depth exploration of the reciprocal cross talk between microglia and the organoid is still needed.
In conclusion, this is a really exciting model to study neuron-microglia communication in vivo and it opens the possibility to perform genetic perturbations at multiple levels to better model human diseases.
References:
Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JC, Sajti E, Jaeger BN, O'Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017 Jun 23;356(6344) Epub 2017 May 25 PubMed.
Mancuso R, Van Den Daele J, Fattorelli N, Wolfs L, Balusu S, Burton O, Liston A, Sierksma A, Fourne Y, Poovathingal S, Arranz-Mendiguren A, Sala Frigerio C, Claes C, Serneels L, Theys T, Perry VH, Verfaillie C, Fiers M, De Strooper B. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci. 2019 Dec;22(12):2111-2116. Epub 2019 Oct 28 PubMed.
Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, McQuade A, Kolahdouzan M, Echeverria K, Claes C, Nakayama T, Azevedo R, Coufal NG, Han CZ, Cummings BJ, Davtyan H, Glass CK, Healy LM, Gandhi SP, Spitale RC, Blurton-Jones M. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron. 2019 Sep 25;103(6):1016-1033.e10. Epub 2019 Jul 30 PubMed.
Xu R, Li X, Boreland AJ, Posyton A, Kwan K, Hart RP, Jiang P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun. 2020 Mar 27;11(1):1577. PubMed.
Balusu S, Horre K, Thrupp N, Snellinx A, Serneels L, Chrysidou I, Arranz AM, Sierksma A, Simren J, Karikari T, Zetterberg H, Chen W-T, Thal DR, Salta E, Fiers M, DeStrooper B. Long noncoding RNA MEG3 activates neuronal necroptosis in Alzheimer's disease. 2022 Feb 20 10.1101/2022.02.18.480849 (version 1) bioRxiv.
Massachusetts General Hospital
Massachusetts General Hospital
Massachusetts General Hospital
This organoid system described by Gage and colleagues is a big step forward in attempts to model AD with human cells. It will now be critical to determine whether these microglia, matured in transplanted human brain organoids, would more closely mimic disease-associated microglia signatures following exposure to Aβ. Previously, the Blurton-Jones lab reported that human iPSC-derived microglia, matured in 5XFAD mouse brains, display human-specific Aβ-responsive genes, and are differentiated from mouse microglia Aβ-responsive genes (Hasselmann et al., 2019) studies, it will be important to know how these new human organoid-matured microglia respond to Aβ accumulation, and how that response compares to microglia found in human AD brains.
References:
Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, McQuade A, Kolahdouzan M, Echeverria K, Claes C, Nakayama T, Azevedo R, Coufal NG, Han CZ, Cummings BJ, Davtyan H, Glass CK, Healy LM, Gandhi SP, Spitale RC, Blurton-Jones M. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron. 2019 Sep 25;103(6):1016-1033.e10. Epub 2019 Jul 30 PubMed.
Make a Comment
To make a comment you must login or register.