Take a Deep Breath—It Just Might Lower Your Risk for Alzheimer’s
Quick Links
Lifestyle changes have been touted as a way to prevent Alzheimer’s disease, but many of these, such as exercising more, are hard to implement. What if prevention were as simple as controlling your breathing? A paper in the March 9 Scientific Reports offers tantalizing hints of this. Researchers led by Mara Mather at the University of Southern California, Los Angeles, found that healthy adults who did a daily slow-breathing exercise for a month lowered the amount of Aβ40 and Aβ42 in their blood. Because high plasma Aβ is associated with AD risk and general mortality, it is possible this drop could be protective, although that remains to be shown. Mather noted that other healthy behaviors, such as exercise or improving sleep, have not yet demonstrated the ability to change Aβ in a favorable direction compared to control interventions. “To have a behavioral intervention that moves the dial on plasma Aβ is really unique,” she told Alzforum.
- A month of slow breathing lowered plasma Aβ40 and Aβ42 in healthy adults.
- In older people, it also nudged up the plasma Aβ42/Aβ40 ratio, which falls in AD.
- Could these effects lessen Alzheimer’s risk over time?
Researchers called the study a good start. “The fact that they saw changes in biomarkers with this relatively mild intervention is quite interesting and exciting. Interpreting those changes is a more complex issue,” Erik Musiek at Washington University in St. Louis wrote to Alzforum. Yonas Geda at the Barrow Neurological Institute in Phoenix agreed. “This is a good pilot study. However, bigger samples measuring at least CSF-derived Aβ and tau species are needed,” he wrote (full comment below).
Why modify breathing? Because it affects heart rate. When people inhale, their heartbeat speeds up, and when they exhale, it slows down. This heart rate variability (HRV) is controlled by the vagus nerve, and is thus an indication of parasympathetic nerve activity. The vagus communicates to all major organs, and it counteracts stimulation from the sympathetic nervous system. It can cause heart rate and blood pressure to drop suddenly, as when a person faints at the sight of blood or from emotional distress. Inhaling partially blocks vagal impulses, speeding up the heart.
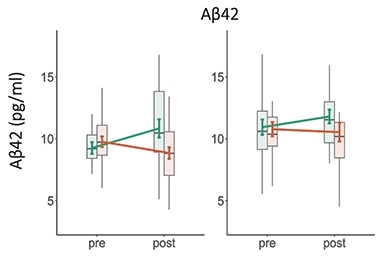
Controlling Plasma Aβ. When younger (left) or older (right) healthy volunteers boosted their heart rate oscillations with biofeedback-guided slow breathing (orange), plasma Aβ42 fell, but when they kept their heart rate steady (green), it rose. The difference may reflect activation of the parasympathetic nervous system in the former condition. [Courtesy of Min et al., Scientific Reports.]
Having narrow HRV is associated with anxiety and depression (e.g., Cheng et al., 2022; Sgoifo et al., 2015). HRV can be modified by breathing at a slow pace, with inhalations and exhalations each lasting about five seconds. This produces large heart rate oscillations, going from a low of about 60 to a high of 80 beats per minute with each breath. Numerous studies have found that artificially raising HRV in this way improves emotional regulation and lessens signs of stress (Goessl et al., 2017; Blase et al., 2021). People who practice yoga and other forms of stress reduction often focus on deep, five-second breaths.
Mather and colleagues previously reported that when healthy volunteers boosted their HRV through biofeedback-guided slow breathing, they strengthened functional connectivity, and even plumped up gray matter, in brain networks associated with emotional regulation, as judged by MRI scans (Nashiro et al., 2022; Yoo et al., 2022).
For the current study, first author Jungwon Min analyzed plasma samples from that biofeedback trial, which comprised 54 young adults with a mean age of 23, and 54 older adults with a mean age of 66. Participants performed biofeedback sessions at home for half an hour each day, clipping a sensor to their earlobes to detect their heart rates, which were displayed on a computer screen. In the experimental condition, they were asked to breathe in a 10-second rhythm, five seconds in and five seconds out, to maximize heart rate oscillations. This produced the characteristic 20 beats per minute swings described above. In the control condition, participants tried to keep their heart rates steady by using relaxation techniques such as listening to calming sounds, while watching their displayed heart rhythm as a guide.
Researchers compared plasma Aβ at baseline and four weeks. In both age groups, Aβ40 and Aβ42 dropped in the experimental condition, and rose in the control condition. The difference was statistically significant. For tau, there was no significant effect in the cohort overall. However, in the young adults, slow breathing suppressed total tau, and in the older adults, it boosted p-tau181. It is unclear what the tau findings mean.
What might explain the Aβ results? The drop likely reflects changes occurring outside the brain, Mather believes. The lower levels could indicate less peripheral Aβ production, since oscillations in blood flow trigger endothelial cells to release factors that suppress platelets, which are the primary source of this peptide in plasma. On the other hand, blood flow variations could be boosting clearance by stimulating the kidneys to dispose of Aβ, she suggested.
In either case, because blood acts as a sink for Aβ cleared from the brain, the lower peripheral levels might indirectly affect CNS abundance, Mather suggested. Some anti-amyloid antibodies were initially hypothesized to work this way, by mopping up Aβ in the blood (Jul 2001 news; Aug 2007 news; Dec 2007 news). One hint of this was that in older adults, who may be starting to accumulate amyloid plaque, the plasma Aβ42/Aβ40 ratio trended larger after four weeks of slow breathing. Since more Aβ42 is produced in the brain than the periphery, and levels of the soluble peptide in the cerebrospinal fluid fall as it gets subsumed by plaques that begin to form, the relative increase in plasma Aβ42 compared to Aβ40 might reflect better clearance from the brain, the scientists suggested. Supporting this, at baseline the older adults had lower Aβ42/Aβ40 and higher p-tau181/total tau than younger adults, suggesting some AD-like changes in their brains.
To parse out these possibilities, the authors are conducting a follow-up biofeedback study on older adults. They will test a nine-week intervention, and will measure platelet activity and Aβ in urine, as a marker of kidney function, to glean clues as to why the peptide falls in the plasma. The researchers will also measure perivascular spaces in the brain via MRI, as a drop there could reflect better drainage of molecules across the blood-brain barrier. Large perivascular spaces have been linked to poor clearance and dementia (Jul 2017 news; May 2016 news; Feb 2022 news).
Assuming the findings replicate, Mather suggested HRV biofeedback early in life might help stave off late-life diseases. HRV is known to plummet as much as 80 percent with age, Mather noted, while sympathetic nervous activity, controlled by the noradrenergic system, rises (Fukusaki et al., 2000; Bonnemeier et al., 2003; Lee et al., 2004). In her study, slow breathing suppressed noradrenergic signaling in the blood as well. Noradrenaline is produced by the locus coeruleus, a site of early tau pathology in the brain (Sep 2019 news; Sep 2021 news).
“Could this age-related change in autonomic activity be one of the factors that accelerates risk of AD?” Mather speculated. “If so, we should be targeting the parasympathetic system and strengthening it as people age.”
Qin Wang at Augusta University, Georgia, agreed the balance between sympathetic and parasympathetic nerve activity could be a factor. “This evidence supports the idea of a causal relationship between noradrenergic activity and the regulation of Alzheimer’s disease hallmarks,” she wrote (full comment below).

All Dressed Up. Transgenic mice with photosensitive receptors in their heart wear a vest fitted with a micro-LED light, allowing researchers to signal their heart rate to speed up or slow down. [Courtesy of Nature.]
Another recent paper adds weight to the idea that altering heart rate can affect the brain. In the March 1 Nature, researchers led by Karl Deisseroth at Stanford University, California, described a mouse optogenetic study. They generated transgenic mice with a photosensitive receptor in cardiomyocytes, then drove heart rhythms by having the mice wear a small vest that delivered light to the chest. When the researchers accelerated heartbeats to 900 per minute, about a third more than the normal rate for a mouse, the animals showed more anxiety than usual, avoiding open spaces. The effect seemed to be mediated by the posterior insular cortex, because blocking activity in this brain region via optogenetics relieved the anxious behavior.
“This is an unequivocal demonstration that, at least in mice, heart rate can affect anxiety, and can probably influence other emotional behavior too,” wrote Yoni Couderc and Anna Beyeler at Bordeaux University, France, in an accompanying News & Views. If slowing the heart rate relieves anxiety, as well, that might help explain changes in brain activity and Aβ level seen with slow breathing.—Madolyn Bowman Rogers
References
News Citations
- Two Ways to Attack Amyloid: Metal Chelator and Antibody
- Enhancing Peripheral Sink Scours Brain of Amyloid
- Without Peripheral Sink, Antibody’s Aβ Clearance up in the Air?
- Ring Around the Vessel: Enlarged Spaces Signal Vascular Disease
- Sleep and Brain Cleansing—Fresh Insights into Regulation and Disruption
- Not Just Aβ—Glymphatic Flow Clears Tau, Too, Slowing Its Aggregation
- Tiny Brain Structure Plays Big Role in Memory
- Is a Waning Locus Coeruleus an Early Sign of Alzheimer’s Disease?
Paper Citations
- Cheng YC, Su MI, Liu CW, Huang YC, Huang WL. Heart rate variability in patients with anxiety disorders: A systematic review and meta-analysis. Psychiatry Clin Neurosci. 2022 Jul;76(7):292-302. Epub 2022 Apr 27 PubMed.
- Sgoifo A, Carnevali L, Alfonso Md, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015;18(3):343-52. Epub 2015 May 25 PubMed.
- Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. 2017 Nov;47(15):2578-2586. Epub 2017 May 8 PubMed.
- Blase K, Vermetten E, Lehrer P, Gevirtz R. Neurophysiological Approach by Self-Control of Your Stress-Related Autonomic Nervous System with Depression, Stress and Anxiety Patients. Int J Environ Res Public Health. 2021 Mar 24;18(7) PubMed.
- Nashiro K, Yoo HJ, Min J, Cho C, Nasseri P, Zhang Y, Lehrer P, Thayer JF, Mather M. Effects of a randomised trial of 5-week heart rate variability biofeedback intervention on mind wandering and associated brain function. Cogn Affect Behav Neurosci. 2022 Dec;22(6):1349-1357. Epub 2022 Jun 27 PubMed.
- Yoo HJ, Nashiro K, Min J, Cho C, Bachman SL, Nasseri P, Porat S, Dutt S, Grigoryan V, Choi P, Thayer JF, Lehrer PM, Chang C, Mather M. Heart rate variability (HRV) changes and cortical volume changes in a randomized trial of five weeks of daily HRV biofeedback in younger and older adults. Int J Psychophysiol. 2022 Nov;181:50-63. Epub 2022 Aug 27 PubMed.
- Fukusaki C, Kawakubo K, Yamamoto Y. Assessment of the primary effect of aging on heart rate variability in humans. Clin Auton Res. 2000 Jun;10(3):123-30. PubMed.
- Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, Katus HA. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003 Aug;14(8):791-9. PubMed.
- Lee PY, Yun AJ, Bazar KA. Conditions of aging as manifestations of sympathetic bias unmasked by loss of parasympathetic function. Med Hypotheses. 2004;62(6):868-70. PubMed.
Further Reading
Primary Papers
- Min J, Rouanet J, Martini AC, Nashiro K, Yoo HJ, Porat S, Cho C, Wan J, Cole SW, Head E, Nation DA, Thayer JF, Mather M. Modulating heart rate oscillation affects plasma amyloid beta and tau levels in younger and older adults. Sci Rep. 2023 Mar 9;13(1):3967. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
I must commend these investigators for integrating plasma AD biomarkers into their study of a behavioral intervention. That they saw changes in these biomarkers with this relatively mild intervention is quite interesting and exciting. Interpreting those changes is a more complex issue. While there is a decrease in plasma Aβ, it's unclear if that is due to decreased production in the brain, increased proteolysis, or decreased clearance from the brain to the blood.
The link to noradrenergic function, which seems a bit tenuous, implicates the glymphatic system. However, one might expect to see higher plasma Aβ levels with increased glymphatic function and clearance from brain to blood. Moreover, the increase in p-tau levels in plasma could be interpreted as a negative. The addition of CSF biomarkers might clarify some of these issues in the future. Despite these questions, it's certainly a thought-provoking study.
Medical College of Georgia at Augusta University
The role of the noradrenergic system in the pathogenesis and progression of Alzheimer's disease has gained increasing attention in recent years. This clinical trial study demonstrated that by simulating vagus nerve pathways through heart rate variability feedback, there were observable changes in the levels of amyloid and tau in plasma. It would be valuable to additionally investigate changes in these biomarkers in cerebrospinal fluid (CSF).
Nonetheless, this evidence in living people supports the idea of a causal relationship between noradrenergic activity and the regulation of Alzheimer’s disease hallmarks.
Make a Comment
To make a comment you must login or register.