Cryo-EM Unveils Distinct Aβ42 Fibril Structures for Sporadic, Familial AD
Quick Links
When asked to name their favorite letter, Aβ fibrils pulled from the brains of 10 people answered with a resounding “S.” This, according to a cryo-electron microscopy study led by Bernardino Ghetti of Indiana University, Indianapolis, and Benjamin Ryskeldi-Falcon, Sjors Scheres, and Michel Goedert of the MRC Laboratory of Molecular Biology, Cambridge, England, U.K. Published January 13 in Science, the study detected two main types of Aβ42 filament. Type I predominated in cases of sporadic AD; they were built with paired Aβ42 protofilaments curved into S shapes, which reached out to hug each other. Type II predominated in cases of familial AD and, surprisingly, were also the only configuration in people with other neurodegenerative diseases and in APP knock-in mice. The core of these filaments also consisted of paired S-shaped protofilaments, but they were arranged differently from those in type I filaments. While the new structures bear resemblance to some previously reported structures of Aβ fibrils, they are novel at their core.
- Cryo-EM of Aβ42 fibrils from 10 brains revealed two predominant filament structures.
- Type I and type II filaments comprised distinct, S-shaped protofilaments.
- Type I filaments predominated in sporadic AD; type II was found in familial AD, related diseases, and in APP knock-in mice.
“The gorgeous and informative cryo-EM images of Aβ assemblies presented by Yang and colleagues are a significant step forward in deciphering the link between protein aggregation and disease,” wrote Mathias Jucker of the German Center for Neurodegenerative Diseases in Tübingen and Lary Walker of Emory University in Atlanta in a comment to Alzforum.
“Congratulations to the authors. At last, we see high-resolution structures of Aβ42-dominated filaments from patient brains,” wrote Dieter Willbold of Heinrich Heine University, Düsseldorf, Germany. “These are highly aesthetic structures that are similar and yet so different, especially in their protofilament arrangement. We will learn a lot more from them.”
Numerous structures of Aβ fibrils have been described using techniques such as NMR and cryo-EM. They differ wildly depending on their source, i.e., whether fibrils were grown in vitro, amplified from fibrils extracted from the brain, or plucked from the brain and viewed as-is (May 2015 news; Jan 2017 news; Sep 2017 news). While fibrils made in vitro tended to twist to the left, as most β-sheet fibrils do, one cryo-EM study led by Marcus Fändrich at Ulm University in Germany found that Aβ fibrils extracted from the meninges of people with AD twisted to the right (Nov 2019 news). Consistent with the known predominance of Aβ40 in vascular Aβ deposits, fibrils from the meninges harbored mostly this peptide. Each folded into a C-shaped structure, arranged back-to-back along the length of the fibril.
How would Aβ fibrils isolated from the brain parenchyma—which largely consist of Aβ42 peptides—compare? Do structures differ depending on the cause of AD? What about Aβ fibrils haunting the brains of people who died with other neurodegenerative diseases? Co-first authors Yang Yang, Diana Arseni, and Wenjuan Zhang and colleagues addressed these questions by focusing their cryo-electron microscope on Aβ fibrils extracted from 10 postmortem brain samples. Five samples came from people with AD, including three with sporadic AD and two with familial AD. The other five came from people with aging-related tau astrogliopathy (ARTAG), Parkinson’s disease dementia (PDD), dementia with Lewy bodies (DLB), familial frontotemporal dementia (FTD) caused by a GRN mutation, and from a 59-year-old man with amyloid deposits who had died of cardiac arrest but did not have dementia.

Type I Filaments. Type I filaments (left) of sporadic AD consist of a pair of Aβ42 protofilaments, each of which forms an S-shaped curve and an extended arm, with which it cradles its neighboring protofilament. Type Ib filaments (right) consist of side-by-side type I filaments, loosely tethered by polar interactions. [Courtesy of Yang et al., Science, 2022.]
What did they see? In people with sporadic AD, they spotted predominantly the left-twisted type I filament. Each protofilament consisted of a pair of Aβ42 peptides, each folded into an S-shaped configuration. The N-terminal segment of each S jutted out, cradling its protofilament partner (see image at right). The core fibril structure comprised five β-strands—two within the extended arm and three within the S itself. Residues 9-18 made up the N-terminal arm, while 19-42 formed the S.
Akin to the fuzzy coat described for tau’s N-terminus, residues 1-8 of Aβ had no consistent structure and were not part of the filament core. The two protofilaments packed against each other using hydrophobic interactions. Type I filaments predominated in all three sporadic AD cases. However, for cases 1 and 3, 13 percent of type I filaments doubled up, forming a side-by-side filament structure that the researchers dubbed type 1b (see image below).

Type II Filaments. These filaments predominated in people with familial AD, and were the only type found in people with other neurodegenerative diseases and in APP knock-in mice. [Courtesy of Yang et al., Science, 2022.]
A different fibril structure predominated in Aβ deposits from people with familial AD. Like type I filaments, these type II filaments also comprised protofilaments of paired Aβ42 peptides striking an S-shaped pose. However, type II S’s were arranged differently, and lacked the N-terminal extension embraces of the type I's. Instead, type II protofilaments held each other at arm’s length, using electrostatic interactions to form a minimal interface free from entanglements. While all fibrils detected in case 1 of familial AD were type II, these fibrils made up 76 percent of the structures in the second case, who also had some type I fibrils. Type II filaments were also detected one case of sporadic AD, where they represented 17 percent of the Aβ fibrils.
Notably, 100 percent of Aβ fibrils detected in people with the other disorders contorted into type II filaments. The same held true for fibrils extracted from APP knock-in mice.
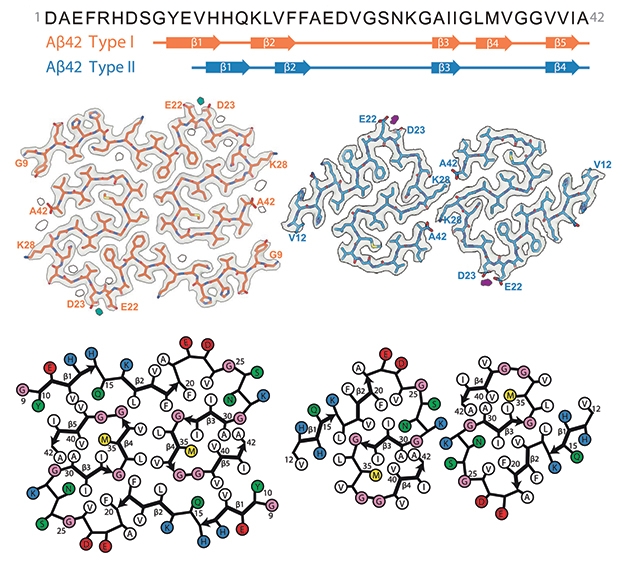
S-Siblings. Aβ42 (sequence at the top) folds into type I and type II filament structures. Both have S-shaped protofilaments, but similarities end there. Protofilaments of each filament type include different amounts of β-strands (top), and are arranged in distinct configurations. [Courtesy of Yang et al., Science, 2022.]
Why do type I fibrils predominate only in sporadic AD? This remains unknown, but the researchers pointed out some notable distinctions among sporadic cases. For one, cases of sporadic AD had more focal plaques with dense cores than did any other disease group, in whom diffuse deposits predominated. People with sporadic AD were oldest when they died. How these factors might tip the balance toward type I filaments remains unclear.
To Goedert, the biggest surprise was that the same exact Aβ filament structure—type II—marked familial AD, the other diseases, pathological aging, and APP knock-in mice. This scenario is in stark contrast to tau, where Goedert and colleagues found distinct protofilament folds in different tauopathies (Oct 2021 news).
These precisely matching Aβ fibril structures appear at odds with studies describing “clouds” of Aβ conformers detected by luminescent conjugated oligothiophenes (LCOs), which suggested that more than one configuration of Aβ fibril exists not only among people with different forms of AD, but even within a single brain (Aug 2017 conference news; Nov 2017 news). Other indirect methods have inferred a similar diversity for tau filament structures in people with AD (Jan 2022 news).
Goedert cannot explain these discrepancies. At least for tau, he said, it is possible that variations in the structure of the fuzzy coat, as opposed to the ordered core of the filament, could explain the structural variation reported by indirect methods. However, compared to tau’s shaggy fuzzy coat, which comprises 80 percent of the protein, Aβ42’s is more like peach fuzz, using only 20 percent of the peptide. Goedert doubts that structural variations in this small part of the peptide entirely explain the clouds of configurations inferred by LCO binding. He also noted that cryo-EM detects upwards of 99 percent of Aβ filament structures, making it unlikely that they simply missed different structures in their analysis.
Jucker and Walker, who led the Aβ LCO study, noted that LCO binding and cryo-EM are fundamentally different techniques. “LCOs (particularly when used in combinations) largely show the arrangement and packing of Aβ-amyloid fibrils; the readout varies with the different Aβ40/42 ratios, and it also is sensitive to age-related changes in the aggregates,” they wrote. Along those lines, Jucker and Walker raised the question of whether Aβ fibril structures might change with age. “While the current study focuses on AD patients with end-stage disease, in which fibrils have been brewing for two to three decades, the seeding activity of Aβ is highest at earlier stages of the pathogenic process,” they wrote.
Robert Tycko, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, wondered if Yang and colleagues may have missed some structures. “Although the cryo-EM-based structures are certainly correct, it is difficult to know whether these are the only, or the predominant, polymorphs in the original tissue, since fibrils extracted from cortical tissue tend to be highly self-associated, entangled with one another, and coated with other material,” he noted. Because the cryo-EM analysis focuses on fibrils that are relatively clean, “a large fraction of the fibril segments end up being discarded,” he wrote (comment below). Goedert acknowledged that they have to discard fibrils that are stuck together, and wondered if this may explain why they didn’t see any Aβ40 fibrils. Using solid-state NMR, Tycko previously found two main polymorphs, but also other forms that showed up in varying intensities from different patients.
How did the new structures compare to older ones? While they are different than the right-twisting C shaped protofilaments of Aβ40 from the meninges, left-handed filaments and S-shaped protofilaments have been reported for Aβ42 fibrils made in vitro. Even so, none exactly matched the configurations described here, noted Fändrich and Michael Willem of Ludwig Maximilians University, Munich, in a perspective in Science. “This is in accordance with previous conclusions that in vitro fibrils do not necessarily model the specific morphologies of ex vivo fibrils,” they wrote. Willem and Fändrich pointed out that the S-shaped fold resembles a conformation previously suggested via NMR for an Aβ42 oligomer (Ahmed et al., 2010). Still, Tycko found the similarities very interesting. “Although the conformations are very similar, the details of some side chain orientations and side chain-side chain interactions are different. This shows how subtle the structural distinctions among amyloid polymorphs can be,” he wrote. “It is amazing how many similar but different structures these peptides can form.”—Jessica Shugart
References
News Citations
- Danger, S-Bends! New Structure for Aβ42 Fibrils Comes into View
- Do Palettes of Aβ Fibril Strains Differ Among Alzheimer’s Subtypes?
- Amyloid-β Fibril Structure Bares All
- Right Turn: Aβ Fibril Structure from Alzheimer’s Brain Reveals Surprising Twist
- Flock of New Folds Fills in Tauopathy Family Tree
- Monomeric Seeds and Oligomeric Clouds—Proteopathy News from AAIC
- Paper Alert: Aβ Fibril Structures Vary by AD Subtype
- Does Fast-Progressing Alzheimer's Have a Whole Repertoire of Tau Conformers?
Research Models Citations
Paper Citations
- Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat Struct Mol Biol. 2010 May;17(5):561-7. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Yang Y, Arseni D, Zhang W, Huang M, Lövestam S, Schweighauser M, Kotecha A, Murzin AG, Peak-Chew SY, Macdonald J, Lavenir I, Garringer HJ, Gelpi E, Newell KL, Kovacs GG, Vidal R, Ghetti B, Ryskeldi-Falcon B, Scheres SH, Goedert M. Cryo-EM structures of amyloid-β 42 filaments from human brains. Science. 2022 Jan 14;375(6577):167-172. Epub 2022 Jan 13 PubMed.
- Willem M, Fändrich M. A molecular view of human amyloid-β folds. Science. 2022 Jan 14;375(6577):147-148. Epub 2022 Jan 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Hertie Institute for Clinical Brain Research, University of Tübingen, and DZNE Tübingen
Emory University
This impressive new research from the Cambridge group supports previous NMR findings of Tycko and coworkers (Lu et al., 2013; Qiang et al., 2017) of a predominant Aβ structure in a particular AD brain. Using amyloid-binding dyes such as luminescent conjugated oligothiophenes (LCOs), we also found that the dense, amyloid cores of plaques are quite similar among cortical regions of a given brain, but that the LCO spectra differ among individuals and among familial and sporadic AD cases (Rasmussen et al., 2017). (Note, however, that the present study likely analyzed some of the same familial brains as those in Rasmussen et al., and thus the findings of the two studies are not completely independent.)
LCOs and cryo-EM do not similarly assess the structural features of polymerized Aβ. LCOs (particularly when used in combinations) largely show the arrangement and packing of Aβ-amyloid fibrils; the readout varies with the different Aβ40/42 ratios, and it also is sensitive to age-related changes in the aggregates (Nyström et al., 2013). LCOs also are not specific for Aβ, in that they bind to a variety of amyloid structures (e.g., Aslund et al., 2009). Using two different LCOs, we found within a given brain polymorphic Aβ-amyloid cores that cluster as clouds around a dominant structure (Rasmussen et al., 2017). Furthermore, the dominant structures of the plaques in human AD cases could be partially recapitulated in experimental mice in our in vivo seeding paradigm.
An important finding of the present and previous (Kollmer et al., 2019) cryo-EM investigations is that synthetic Aβ fibrils have a different structure compared to brain-derived Aβ fibrils. This observation urges caution in the interpretation of analyses of synthetic Aβ assemblies, and it may explain why synthetic Aβ is a poor seed compared to brain-derived material (Meyer-Luehmann et al., 2006). However, whether this poor seeding is simply based on the molecular structure of the assemblies or on brain-derived cofactors is still an open question. It will be exciting to investigate the comparative seeding potential of Aβ fibrils that have been well-characterized by cryo-EM in animal models.
The gorgeous and informative cryo-EM images of Aβ assemblies presented by Yang and colleagues are a significant step forward in deciphering the link between protein aggregation and disease. As always in biology, such nice findings also raise more questions. For example, what role might age alone play in defining the molecular architecture of Aβ fibrils? The present analysis focuses on AD patients with end-stage disease, at least two (maybe three) decades after Aβ deposition has started in the brain; the highest specific Aβ seeding activity, however, has been found at early stages of the pathogenic process (Aoyagi et al., 2019; Ye et al., 2017).
Does the sarkosyl extraction procedure favor the isolation of specific Aβ structures? It will be useful to know how mutations that alter the amino acid sequence of Aβ influence the structure of the fibrils (and, for that matter, how mutations outside of Aβ—in APP and presenilin—manage to promote different structures). And how do Aβ40, Aβ42 and other variants interact in fibril formation, and what are the consequences for disease? Does fibril structure influence the sensitivity of imaging agents (or assays) for quantifying Aβ load in the brain? By furnishing new insights into protein aggregation, cryo-EM will be highly useful in addressing these and other open questions in the field.
References:
Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular Structure of β-Amyloid Fibrils in Alzheimer's Disease Brain Tissue. Cell. 2013 Sep 12;154(6):1257-68. PubMed.
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes. Nature. 2017 Jan 12;541(7636):217-221. Epub 2017 Jan 4 PubMed.
Rasmussen J, Mahler J, Beschorner N, Kaeser SA, Häsler LM, Baumann F, Nyström S, Portelius E, Blennow K, Lashley T, Fox NC, Sepulveda-Falla D, Glatzel M, Oblak AL, Ghetti B, Nilsson KP, Hammarström P, Staufenbiel M, Walker LC, Jucker M. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer's disease. Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):13018-13023. Epub 2017 Nov 20 PubMed.
Nyström S, Psonka-Antonczyk KM, Ellingsen PG, Johansson LB, Reitan N, Handrick S, Prokop S, Heppner FL, Wegenast-Braun BM, Jucker M, Lindgren M, Stokke BT, Hammarström P, Nilsson KP. Evidence for Age-Dependent in Vivo Conformational Rearrangement within Aβ Amyloid Deposits. ACS Chem Biol. 2013 Mar 29; PubMed.
Aslund A, Sigurdson CJ, Klingstedt T, Grathwohl S, Bolmont T, Dickstein DL, Glimsdal E, Prokop S, Lindgren M, Konradsson P, Holtzman DM, Hof PR, Heppner FL, Gandy S, Jucker M, Aguzzi A, Hammarström P, Nilsson KP. Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol. 2009 Aug 21;4(8):673-84. PubMed.
Rasmussen J, Mahler J, Beschorner N, Kaeser SA, Häsler LM, Baumann F, Nyström S, Portelius E, Blennow K, Lashley T, Fox NC, Sepulveda-Falla D, Glatzel M, Oblak AL, Ghetti B, Nilsson KP, Hammarström P, Staufenbiel M, Walker LC, Jucker M. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer's disease. Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):13018-13023. Epub 2017 Nov 20 PubMed.
Kollmer M, Close W, Funk L, Rasmussen J, Bsoul A, Schierhorn A, Schmidt M, Sigurdson CJ, Jucker M, Fändrich M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer's brain tissue. Nat Commun. 2019 Oct 29;10(1):4760. PubMed.
Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, Vigouret JM, Paganetti P, Walsh DM, Mathews PM, Ghiso J, Staufenbiel M, Walker LC, Jucker M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006 Sep 22;313(5794):1781-4. PubMed.
Aoyagi A, Condello C, Stöhr J, Yue W, Rivera BM, Lee JC, Woerman AL, Halliday G, van Duinen S, Ingelsson M, Lannfelt L, Graff C, Bird TD, Keene CD, Seeley WW, DeGrado WF, Prusiner SB. Aβ and tau prion-like activities decline with longevity in the Alzheimer's disease human brain. Sci Transl Med. 2019 May 1;11(490) PubMed.
Ye L, Rasmussen J, Kaeser SA, Marzesco AM, Obermüller U, Mahler J, Schelle J, Odenthal J, Krüger C, Fritschi SK, Walker LC, Staufenbiel M, Baumann F, Jucker M. Aβ seeding potency peaks in the early stages of cerebral β-amyloidosis. EMBO Rep. 2017 Sep;18(9):1536-1544. Epub 2017 Jul 12 PubMed.
University of California, Irvine
This is an interesting and important study. The authors report the first structure of Aβ42 fibrils from the brain and although there have been other reports of Aβ42 fibril structures assembled from monomer in vitro, previous work has indicated that the structures of fibrils isolated from brain are quite different.
Another interesting insight is that the authors observed only two different structures in 10 human brain samples from a range of disease phenotypes, including people with familial AD (FAD) mutations. Type I is found in sporadic AD (sAD), while type II is a minor component in sAD and the dominant form in FAD. Type II was found exclusively in a variety of different disease subtypes, such as PDD, DLB, and FTD. For tau, α-synuclein, and TDP43 amyloids, different structural polymorphs are associated with disease subtypes, and with many disease subtypes in AD, it is possible that there may have been more structures found, which would complicate strategies to image and target the different structures.
Other human brain amyloid structures have been reported from the Tycko (Ghosh et al., 2021) and Fändrich (Kollmer et al., 2019) laboratories, and Collinge and Tycko (Ghosh et al., 2021; Qiang et al., 2017) have published evidence that fibrils from non-demented individuals and fibrils from the rapidly progressing form of AD display subtle structural differences by solid-state NMR compared to fibrils from sAD. It is possible that these distinct structures in human brain are associated with different brain locations and disease phenotypes than the ones sampled by Scheres, Goedert, and co-workers, but it is also possible that some of the differences are due to methodology. In order to observe single fibrils in cryo-EM, Scheres and Goedert used Sarkosyl detergent extraction. Tycko and Fändrich used milder, less denaturing conditions, but in order to observe single fibrils Tycko used sonicated brain amyloid to seed Aβ40 monomer addition. Fändrich used amyloid isolated from leptomeninges, which is more soluble. It is interesting that several of the structures are very similar in the region between amino acids 25-35, suggesting that this region is the least polymorphic. Ironically this also represents the “toxic domain” that was the poor man’s Aβ substitute and widely used in the 1990s (Behl et al., 1992).
Amyloid deposits in human brain show differences in monoclonal antibody and fluorescent dye binding. If there are only two structures in sAD, then differences in dye and antibody binding are either due to polymorphic structures in the amino-terminal “fuzzy coats” (residues 1-12) of the type I and type II structures, and of many in vitro structures that are insufficiently ordered to be discerned in the cryoEM structure. Alternatively, dye and antibody bonding may be influenced by other proteins or molecules that associate with fibrils. This amino terminal domain contains the major binding site for most anti-Aβ antibodies. These antibodies recognize different epitopes and have different binding modes to the same sequence within this region, suggesting that this region can adopt different structures in vivo (Reyes-Ruiz et al., 2020).
The big questions remaining include: What structures do brain Aβ oligomers adopt? Are they distinct from that of fibril protofilaments? The most obvious possibility is that some oligomers could be small pieces of the fibrils or fibril protofilaments (Wu et al., 2010), but there is immunological evidence that other structural classes of oligomers also exist in brain (Glabe, 2008).
References:
Ghosh U, Thurber KR, Yau WM, Tycko R. Molecular structure of a prevalent amyloid-β fibril polymorph from Alzheimer's disease brain tissue. Proc Natl Acad Sci U S A. 2021 Jan 26;118(4) PubMed.
Kollmer M, Close W, Funk L, Rasmussen J, Bsoul A, Schierhorn A, Schmidt M, Sigurdson CJ, Jucker M, Fändrich M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer's brain tissue. Nat Commun. 2019 Oct 29;10(1):4760. PubMed.
Ghosh U, Yau WM, Collinge J, Tycko R. Structural differences in amyloid-β fibrils from brains of nondemented elderly individuals and Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2021 Nov 9;118(45) PubMed.
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes. Nature. 2017 Jan 12;541(7636):217-221. Epub 2017 Jan 4 PubMed.
Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992 Jul 31;186(2):944-50. PubMed.
Reyes-Ruiz JM, Nakajima R, Baghallab I, Goldschmidt L, Sosna J, Ho PN, Kumosani T, Felgner PL, Glabe CG. An "epitomic" analysis of the specificity of conformation-dependent, anti-Aß amyloid monoclonal antibodies. J Biol Chem. 2020 Dec 9; PubMed.
Wu JW, Breydo L, Isas JM, Lee J, Kuznetsov YG, Langen R, Glabe C. Fibrillar oligomers nucleate the oligomerization of monomeric amyloid beta but do not seed fibril formation. J Biol Chem. 2010 Feb 26;285(9):6071-9. PubMed.
Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008 Oct 31;283(44):29639-43. PubMed.
NIDDK, NIH
This is excellent work, demonstrating the ability of cryo-EM to determine detailed molecular structures of 42-residue Aβ fibrils that were extracted directly from cortical tissue. The close resemblance of peptide conformations in these brain-extracted fibrils to conformations in “synthetic” 42-residue Aβ fibrils, determined six or seven years ago by the Ishii, Meier, and Griffin labs with solid-state NMR methods, is especially interesting to me. Although the conformations are very similar, the details of some side chain orientations and side chain-side chain interactions are different. This shows how subtle the structural distinctions among amyloid polymorphs can be. It is amazing how many similar but different structures these peptides can form.
One caveat is worth considering: Although the cryo-EM-based structures are certainly correct, it is difficult to know whether these are the only or the predominant polymorphs in the original tissue, since fibrils extracted from cortical tissue tend to be highly self-associated, entangled with one another, and coated with other material. The cryo-EM analysis focuses on fibrils that are relatively “clean,” and a large fraction of the fibril segments end up being discarded. Therefore, the relative abundances in the original tissue cannot be inferred readily from the cryo-EM results. In my lab’s earlier studies of 42-residue Aβ fibrils from cortical tissue of Alzheimer’s disease patients, which were based on solid-state NMR spectra of isotopically labeled fibrils that were seeded with amyloid-containing tissue extracts, we concluded that there were two predominant 42-residue polymorphs, possibly related to the two polymorphs in this paper from Goedert and coworkers (Qiang et al., 2017). However, the solid-state NMR spectra also showed signals from other polymorphs, with variable intensities in samples from different patients.
References:
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes. Nature. 2017 Jan 12;541(7636):217-221. Epub 2017 Jan 4 PubMed.
University of Melbourne
Florey Instotute of Neurosciece and Mental Health
Where’s Wally?
The avalanche of cryo-EM studies in neurodegenerative diseases continues. This latest offering from the Goedert laboratory highlights the S-shaped protofilament fold in Aβ extracted from human brain tissue. The method of extraction appears important, as they report that a modified sarkosyl extract (adding sarkosyl following homogenization), used previously for isolating α-synuclein, provides “abundant Aβ filaments alongside other amyloids,” whereas a more standard sarkosyl extract (adding sarkosyl at later stages) yields “only tau filaments.” This may suggests that sarkosyl extraction protocols are less reproducible than expected, even though there are reports (e.g, Diner et al., 2017) that show that the “sarkosyl in homogenate” approach gives a very similar result as a traditional multistep approach.
The bigger issue is how to select an Aβ fibril as opposed to a tau fibril for cryo-EM analysis. Typical neurofibrillary tangle (NFT) paired helical filaments (PHF) are clearly distinguishable from single straight or twisted filaments. These straight filaments measuring 10 nm are more likely to be Aβ amyloid than tau tangles/neurites, at least based on standard electron microscopy of fixed sectioned brain. We know that a sarkosyl preparation contains a large amount of Aβ as well as tau (mostly N-terminally truncated). Looking at a negatively stained preparation of a sarkosyl extract then becomes a bit of a “Where’s Wally?” issue. Which structures are tau, which are Aβ, and what’s in all the non-filamentous aggregates in the background that are rarely mentioned? Be that as it may, the cryo-EM images (Fig S1A) look like entirely ~10 nm thick Aβ42 filaments, but concurrent negative EM immunostains showed (Fig S8) filaments of >20 nm diameter according to the 50 nm scale bar and 10 nm gold particles. Are these tau filaments with fuzzy Aβ coats decorated with anti-Aβ antibodies?
Notwithstanding these issues, the study describes for the first time the structure of the core (9/12-42) of Aβ42 fibrils extracted directly from 10 postmortem brain samples, including those from subjects who died with sporadic AD, familial AD, or other forms of neurodegenerative disease or aging with AD comorbidity. The authors identified two distinct types of fibril structures, each featuring essentially the same S-shaped core protofibrils, similar to those observed before in in vitro experiments (Fig 4B). However, protofilaments were assembled into fibrils differently. While Type I fibrils predominated in subjects with sporadic AD, type II fibrils predominated in those with familial AD as well as other neurodegenerative diseases. Both fibrillar structures differed from those of fibrils assembled in vitro. Interestingly, APP knock-in mice had Aβ fibrils with a type II structure.
Since the ordered cores of amyloids are assumed to provide templates for autocatalytic growth, the structures of the ordered cores of amyloids may define “conformers” (Alzforum Comments: What is a conformer? by Scheres and Goedert, 07 Jan 2020). Considering the S-shape as part of the topology of a Greek key motif, we have noticed that this Greek key motif is a key element observed so far in all transmissible amyloids including Aβ42, α-synuclein (Li et al., 2018; Tuttle et al., 2016), and PrP (Kraus et al., 2021). We also found elements of this “transmissible conformer” in the “oligomeric” prefibrillar structure of the Aβ17-42 tetramer (Streltsov et al., 2011). But it has not been seen in any of the secondary tau cryo-EM structures published to date. This idea is seen in the figure below.
References:
Diner I, Nguyen T, Seyfried NT. Enrichment of Detergent-insoluble Protein Aggregates from Human Postmortem Brain. J Vis Exp. 2017 Oct 24;(128) PubMed.
Kraus A, Hoyt F, Schwartz CL, Hansen B, Artikis E, Hughson AG, Raymond GJ, Race B, Baron GS, Caughey B. High-resolution structure and strain comparison of infectious mammalian prions. Mol Cell. 2021 Nov 4;81(21):4540-4551.e6. Epub 2021 Aug 25 PubMed.
Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G, Sawaya MR, Shin WS, Boyer DR, Ye S, Eisenberg DS, Zhou ZH, Jiang L. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun. 2018 Sep 6;9(1):3609. PubMed.
Streltsov VA, Varghese JN, Masters CL, Nuttall SD. Crystal structure of the amyloid-β p3 fragment provides a model for oligomer formation in Alzheimer's disease. J Neurosci. 2011 Jan 26;31(4):1419-26. PubMed.
Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM, Rienstra CM. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol. 2016 May;23(5):409-15. Epub 2016 Mar 28 PubMed.
Stony Brook University
For me, one of the still largely unanswered questions in the Alzheimer’s field is how the addition of two amino acids at the C-terminus of the Aβ peptide results in such a dramatic difference in the progression of AD.
Aβ42 predominates in the parenchymal plaques in AD patients, while the more abundant Aβ40 is either cleared from the brain or forms amyloid deposits on or near the cerebral vasculature. The structures of fibrils that have emerged of the Aβ40 and Aβ42 peptides, both in vitro and in vivo, have consistently shown that the additional two amino acids allow the formation of a third β-strand and “S-shaped” structure for Aβ42. So, in this sense, the new brain-derived fibril structures are not so different from the in vitro fibrils.
One of the striking observations in the current brain-derived Aβ42 structures of Yang et al. is that while the S-shaped structure exists, it is different in packing and electrostatic interactions compared to the in vitro structures determined by cryo-EM and NMR. One common feature of the in vitro structures has been a direct electrostatic interaction between the carboxylate of the C-terminal Ala42 and the positively charged Lys28 side chain. Electrostatic interactions between Lys28 and either Glu22 or Asp23 are often observed in Aβ40 fibrils (both in vitro and in vivo) suggesting that this difference in part drives the different folding patterns between Aβ40 and Aβ42.
The observation of a possible bound metal ion at Glu22-Asp23 in the brain-derived fibrils described by Yang et al. may in part be relevant for how these fibrils fold and, more generally, for the role of metal ions in influencing fibril morphology. These observations then raise the importance of both the environment in which the Aβ peptides fold and possible co-factors that influence folding.
BioArctic
BioArctic
Uppsala University
This is an interesting study from the laboratories of Michel Goedert and collaborators, where the authors, Yang et al., describe molecular structures of Aβ42 filaments in human brain. The structures of Aβ42 filaments in brain have not been well-defined. Here, Aβ42 filaments taken postmortem from the brains of 10 patients were analyzed at high resolution using cryo–electron microscopy (cryo-EM). Five individuals had Alzheimer’s disease, with three sporadic and two familial cases, and the other five individuals had other forms of neurodegenerative disease. Two structurally related S-shaped protofilament folds were identified and classified as two different types. Type I filaments were found primarily in the brains of individuals with sporadic Alzheimer’s disease, while type II filaments were identified in individuals with familial Alzheimer’s disease and other forms of neurodegenerative disease.
The study shows that both type I and II structures differed from those of fibrils produced in vitro. This observation strongly suggests, maybe as expected, that the environment has a major impact on the folding pathway from the monomeric state to fibrillar Aβ forms. Even different in vivo conditions (e.g., sporadic versus familial Alzheimer’s disease) seem to have a direct impact on the final fibrillar forms of Aβ in neurodegenerative disease.
Intriguingly, the AppNL-F knock-in mouse model, which expresses humanized Aβ, was shown to possess Aβ identical to type II filaments isolated from human brains. However, it might be too early to tell whether this preclinical Alzheimer’s disease model is more representative of familial Alzheimer’s disease based on these data alone. In addition to the AppNL-F knock-in model, it would be of great value to characterize the Aβ fibril species in other commonly used Alzheimer’s disease models. Observations like these may have important translational value in developing anti-Aβ agents.
Yang et al. focus on the sarkosyl-insoluble fraction of Aβ from human brain. As sarkosyl is stringent enough to solubilize most folded proteins in the brain, less robust oligomeric forms of misfolded Aβ might be lost. Thus, different types of soluble Aβ forms, such as oligomers and protofibrils, could be excluded in the preparation and thus not subject to investigations in this cryo-EM study. Different studies suggest that soluble Aβ forms such as oligomers and protofibrils may represent the primary pathological species in Alzheimer’s disease (Nilsberth et al., 2001; Walsh et al., 2002). These species have proven notoriously difficult to investigate in detail with currently available technologies. The dynamics, stability, and transient lifetime of these soluble toxic species hamper the possibility to precisely pinpoint the toxic structural aspects.
These types of studies could, in the future, add to our understanding of how the different binding profiles of clinical investigated anti-Aβ agents exercise their therapeutic effect. However, it is important to appreciate that these described Aβ structures probably represent a small fraction of all forms of Aβ in the human brain. They most likely belong to the forms of Aβ late in the aggregation pathway.
These identified structures of Aβ from the human brain could also be used to assess the binding profiles of different Aβ PET tracers, and to develop new PET ligands. For instance, it is speculated that the PET tracer PiB probably labels both type I and II filaments as it visualizes Aβ deposits in both sporadic and familial Alzheimer’s disease. It is worth remembering that the PET ligand PiB does not detect the diffuse plaques in brains of the Arctic Alzheimer’s disease mutation carriers, where glutamic acid is changed for glycine in position 22 of Aβ, leading to a propensity to form Aβ protofibrils and Alzheimer’s disease (Schöll et al., 2012).
A bit surprising is that there were no major differences described between the five Alzheimer’s disease cases and the five cases with other neurodegenerative diseases. In the latter five cases, Aβ levels should be much lower in the brain. This does not seem to have an impact on the cryo-EM findings.
References:
Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Näslund J, Lannfelt L. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001 Sep;4(9):887-93. PubMed.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002 Apr 4;416(6880):535-9. PubMed.
Schöll M, Wall A, Thordardottir S, Ferreira D, Bogdanovic N, Långström B, Almkvist O, Graff C, Nordberg A. Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology. 2012 Jul 17;79(3):229-36. PubMed.
Make a Comment
To make a comment you must login or register.