Map of Human Vascular Expression Highlights its Potential Role in Alzheimer’s
Quick Links
Brain health depends on a robust vasculature, but because it's difficult to isolate cerebrovascular cells, scientists still know little about these cell types or how they might change in diseases such as Alzheimer’s. Now, in a preprint posted to bioRxiv, researchers led by Tony Wyss-Coray at Stanford University, Palo Alto, California, describe a new method for isolating vascular and perivascular cells from human brain that is compatible with single-nuclei RNA-Seq. Using the latter, the researchers mapped the gene expression profiles of endothelial and smooth muscle cells located on arteries, capillaries, and veins of healthy aging brain. They also identified distinct subtypes of pericytes and fibroblasts.
- Single-nuclei RNA-Seq of human brain vasculature identifies two subtypes of both pericytes and fibroblasts.
- Dozens of AD risk genes are highly expressed in vascular cells.
- snRNA-Seq of mouse choroid plexus finds inflammation goes up with age.
In AD brain samples, their analysis found numerous gene expression changes compared to healthy brain, and confirmed a massive loss of vascular cell types, particularly those responsible for maintaining the extracellular matrix. Intriguingly, vascular cells expressed 30 of the top 45 AD risk genes, hinting that these understudied cells may play a larger role in pathogenesis than was previously thought. “We need to explore the risk contribution of these different cell types to AD,” Wyss-Coray noted.
Others were enthusiastic. “This is an exciting story from Wyss-Coray’s lab, as now we finally have a beautiful atlas of endothelial and stromal cells from human brain and meninges,” Jonathan Kipnis at Washington University in St. Louis wrote to Alzforum (full comment below). Mark Fiers at KU Leuven, Belgium, called it an important paper for the field. “These results shed a different light on many AD-relevant genes,” Fiers noted.
Meanwhile, researchers led by Maria Lehtinen at Boston Children’s Hospital tackled another understudied brain tissue, the choroid plexus. This tissue lines ventricles and produces the cerebrospinal fluid that bathes the brain. CSF harbors key biomarkers of Alzheimer’s disease. In the April 27 Cell, the researchers delineated a map of gene expression in this mouse choroid plexus, showing how profiles changed across ventricles and with age. In particular, inflammatory signaling ramped up there in old mice.
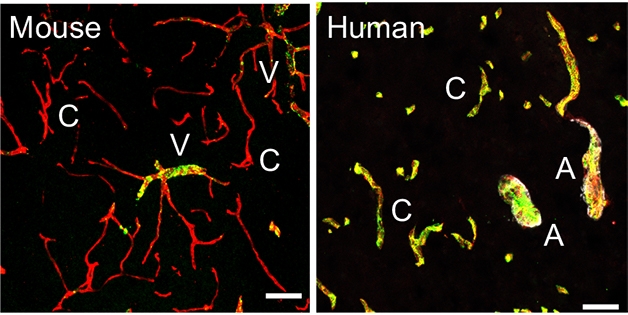
The People vs. Mice. The gene for the von Willebrand clotting factor (yellow) is expressed only in veins (V) in mouse brain (left), but is also expressed in capillaries (C) and arteries (A) in human brain (right). [Courtesy of Yang et al., 2021.]
Previously, researchers led by Christer Betsholtz at Uppsala University, Sweden, had detailed single-cell RNA-Seq maps of mouse brain vasculature. Betsholtz reported a gradual shift in the expression profile of endothelial cells along the arteriovenous continuum, i.e., the progression of the vasculature from arteries to capillaries to veins. By contrast, the delineations between different smooth muscle cell (SMC) and pericyte populations were sharp (Vanlandewijck et al., 2018).
Wyss-Coray and colleagues wanted to extend this work to human brain, but first had to develop a way to isolate intact vascular cell nuclei. This is challenging because of the fibrous extracellular matrix (ECM) surrounding blood vessels. First author Andrew Yang came up with a simple solution that would make any chef proud: He forced it through a sieve. Yang started with 25 frozen, unfixed postmortem sections from the hippocampi and cortices of nine AD patients and eight age-matched controls. After homogenizing the tissue and spinning down vascular fragments, he resuspended the pellet and washed off contaminating parenchymal and blood cells. To free cells from the ECM, he mashed the tissue through a strainer using the flat end of a syringe plunger. Freed cells were lysed to release nuclei. This physical dissociation, dubbed Vessel Isolation and Nuclei Extraction for Sequencing (VINE-Seq), worked better than enzymatic or chemical methods and was less damaging to nuclei, the authors found. From the 25 brain sections VINE-Seq produced 143,793 nuclei for RNA-Seq, more than 100 times the number of vascular cells analyzed in previous snRNA-Seq studies of human brain.
“The new VINE-Seq method that was developed to sequence endothelial cells is pretty remarkable,” Kipnis noted.
Of these 143,793 cells, 36,825, or about one-quarter, were endothelial cells, which line the inside of blood vessels. The authors used a clustering algorithm to arrange these cells along the arteriovenous cell type axis, aided by known markers of arterial, capillary, and venous cells. The data revealed seven gradually differing expression patterns as cell origin shifted from arteries to veins. The continuum was similar to that seen in mice, but many of the specific genetic markers were different. For example, the blood clotting gene von Willebrand factor is only expressed by venous endothelial cells in mice, but is present throughout the vasculature in humans (see image above). The gene expression findings agreed with protein localization in the vasculature as detailed in the Human Protein Atlas (Uhlén et al., 2015).

Distinct Types. Mural cells found on blood vessels in human brain group into four distinct types: arterial smooth muscle cells (aSMC), arteriolar SMCs (aaSMC), matrix-specialized pericytes (M-pericytes), and transport-specialized pericytes (T-pericytes). [Courtesy of Yang et al., 2021.]
On the other hand, among the 34,508 isolated SMCs and pericytes, collectively known as mural cells because they line the walls of the vessels, the authors found distinct profiles that distinguished SMCs on arteries from other contractile cells on arterioles. The field has debated whether the arteriole contractile cells are more akin to SMCs or pericytes, the tiny cells that perch on capillaries (Jun 2015 news; Feb 2017 news; May 2017 news). Wyss-Coray’s data may slice through this Gordonian knot by deeming arteriolar mural cells distinct from both arterial SMCs and from pericytes.
Speaking of pericytes, the authors identified two subtypes of these using expression clustering (see image above). One, which the authors called matrix pericytes (M-pericytes), express genes involved in ECM formation and regulation, while the other, dubbed transport pericytes (T-pericytes), specialize in transmembrane transporters. The authors localized these mural cells along the vasculature using in situ hybridization. Both types of pericytes were found together along capillaries and veins, suggesting they perform essential functions needed throughout the vasculature. Curiously, mural cells on veins exhibited a pericyte expression pattern, with little resemblance to arterial SMCs. As with endothelial cells, the data from human mural cells varied greatly from mouse vasculature, suggesting that mice may not be good models for vascular disorders.
The authors also isolated fibroblasts from around blood vessels. As with pericytes, these fell into two distinct populations, but unlike pericytes, they segregated spatially. One type lurked near blood vessels, the other within the meninges that encase the brain. The 2,985 perivascular fibroblast nuclei they isolated expressed many ECM proteins and also cellular efflux pumps to rid the cells of solutes. In contrast, the 428 meningeal fibroblasts had more transporters that bring solutes into the cell. The authors speculated that this specialized division of inflow and outflow pumping might help drive the glymphatic flow of cerebrospinal fluid through the brain (Aug 2012 news; May 2014 conference news).

Vascular AD Risk. Many of the top AD risk genes (left column) have their highest expression in vascular cell types (listed on top). Numbers on bottom indicate the number of GWAS genes expressed in each cell type; the data in the five right-hand columns are from prior studies. [Courtesy of Yang et al., 2021.]
Is the AD brain any different from age-matched controls? Using VINE-Seq, the authors were able to isolate only about half as many endothelial cells, SMCs, and perivascular fibroblasts from AD brain as from normal brain, and hardly any M-pericytes. T-pericytes, on the other hand, were unchanged. These losses were confirmed by in situ immunostaining with vascular cell marker genes.
Whether these cells are truly missing or have taken on a different gene expression profile is unclear. Gene expression analysis found 463 differentially expressed genes among the AD cells, with mural cells having the most changes compared to age-matched control mural cells, particularly in genes that control vasoconstriction and blood flow. Notably, these AD-related changes showed little overlap with endothelial cell expression changes seen in mouse models of amyloidosis, again suggesting mice do not effectively model AD vascular pathology.
AD risk genes had a large footprint in vascular cell types. In addition to expressing 30 of the top 45 GWAS hits, these cells expressed 383 of 651 genes in a larger AD GWAS list. Several AD genes were expressed more strongly in vascular cells than in microglia, astrocytes, or neurons. For example, ACE is most highly expressed by arterial endothelial cells, and MS4A6A and CR1 by perivascular macrophages. ADAMTS4 finds its strongest expression in SMCs, and FHL2 in perivascular fibroblasts (see image above).
“This should prompt us to look more closely at the role of the vasculature in AD,” Wyss-Coray said. He noted that the majority of AD brains have vascular pathology. The expression data from this study are available in a searchable web interface.
By contrast, the paper by Lehtinen and colleagues also presents a spatial expression map of a type of brain tissue, but this one is for mouse choroid plexus. Joint first authors Neil Dani and Rebecca Herbst microdissected the tissue from the lateral, third, and fourth ventricles of embryonic mice, 4-month-old adult mice, and 20-month-old aged mice. Altogether, they obtained 98,660 nuclei for snRNA-Seq. This set included nuclei from epithelial cells that line ventricles, endothelial and mural cells from blood vessels, fibroblasts, glia, and neurons. To localize cells, the authors combined gene expression findings with immunostaining of marker genes in explants and tissue slices. Their analysis revealed ventricle-specific gene expression patterns, and this regionalization increased with age.
Numerous other genes changed with age, the scientists report. For example, the longevity factor klotho rose in epithelial cells, while the pro-inflammatory cytokine IL-1β spiked in macrophages. Its receptor, IL1R1, went up in numerous choroid plexus cell types, including fibroblasts, epithelial, endothelial, and mural cells. The findings build on previous reports of increased inflammation in the choroid plexus with age, the authors noted (Baruch et al., 2014; Paré et al., 2018).
“This is a very impressive piece of work, and I think it will be of tremendous value for the field,” Wyss-Coray said. He praised the use of mice of different ages and the detailed spatial data as strengths of the study. The next step will be to study choroid plexus in human brain, in health, aging, and disease.—Madolyn Bowman Rogers
References
News Citations
- Smooth Muscle Cells, Not Pericytes, Control Brain Blood Flow
- Pericytes Don’t Go With the Flow—They Change It
- Finally, a Dye to Visualize Pericyte Function
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- Glymphatic Flow, Sleep, microRNA Are Frontiers in Alzheimer’s Research
Paper Citations
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018 Feb 14; PubMed.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419. PubMed.
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014 Aug 21; PubMed.
- Paré A, Mailhot B, Lévesque SA, Juzwik C, Ignatius Arokia Doss PM, Lécuyer MA, Prat A, Rangachari M, Fournier A, Lacroix S. IL-1β enables CNS access to CCR2hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc Natl Acad Sci U S A. 2018 Feb 6;115(6):E1194-E1203. Epub 2018 Jan 22 PubMed.
External Citations
Further Reading
News
- Ruffles and Sphincters Control the Spigot of Fresh Blood in the Brain
- Tunneling Nanotubes—How Pericytes Control Blood Flow
- Do Endothelial Cells Spur Capillaries to Grow in Alzheimer’s Brain?
- Blood-Brain Barrier Surprise: Proteins Flood into Young Brain
- Human Blood-Brain Barrier Model Blames Pericytes for CAA
- Vascular Dysfunction Taxes Cognition, but Not Via Amyloid, AD
- Does Anti-Amyloid Immunotherapy Need the Lymphatic System?
Primary Papers
- Yang AC, Vest RT, Kern F, Lee DP, Maat CA, Losada PM, Chen MB, Agam M, Schaum N, Khoury N, Calcuttawala K, Pálovics R, Shin A, Wang EY, Luo J, Gate D, Siegenthaler JA, McNerney MW, Keller A, Wyss-Coray T. A human brain vascular atlas reveals diverse cell mediators of Alzheimer’s disease risk. bioRxiv. 2021 Apr 27 bioRxiv.
- Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D, Nguyen L, Rodman C, Riesenfeld SJ, Prochazka J, Prochazkova M, Sedlacek R, Zhang F, Bryja V, Rozenblatt-Rosen O, Habib N, Regev A, Lehtinen MK. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021 Apr 27; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis, School of Medicine
This is an exciting story from Wyss-Coray’s lab, as now we finally have a beautiful atlas of endothelial and stromal cells from human brain and meninges.
The diversity of the populations and the changes they observe with AD are very interesting. The fact that many of the AD-associated genes are expressed in endothelial cells, whereas in mice these are more in microglial cells, is another interesting aspect of the paper. We may not fully understand yet what this means, but these results are fascinating enough to dig deeper. I must also note that the new method that was developed here to sequence endothelial cells (VINE-Seq) is pretty remarkable.
KU Leuven
This is a very interesting and thorough paper. They authors describe a massive database of single-cell transcriptomes of AD patients and controls. I much appreciate how they focus on the vasculature, an aspect that does not get enough attention yet, more specifically, in most human AD single-cell studies coming out these days. It is interesting to see that a large number of potentially relevant AD risk genes express in the brain vasculature, and that some of them even differentially express in AD.
However, as with all papers of this scope, we only get to see the tip of the iceberg. I would be very interested to see a deeper analysis of the impact of carrying APOE4 on the gene-expression alterations in AD, in particular in the light of the AD risk genes, as all these aspects are now treated separately.
Just as other papers omit cells from the vasculature, this paper depletes non-vasculature cells, hence I’m not fully convinced of their conclusion that many AD GWAS risk genes actually enrich in the vasculature. However, these results do shed a different light on many AD-relevant genes, including well-known ones such as APOE and PICALM.
A further strong point of the paper is the effort the authors make to highlight the differences between mouse and humans, and to emphasize the care we need to take to interpret results derived from mouse experiments in the light of Alzheimer’s disease.
All combined, I think this is an important paper for the field.
Make a Comment
To make a comment you must login or register.