Aβ in Lewy Body Disease: Two Diseases at Once, or Another Beast Entirely?
Quick Links
Many people with Lewy body diseases (LBDs) such as Parkinson’s ultimately develop dementia, and many have Aβ plaques and tau tangles. Do they have two diseases at the same time, or is this combination of scourges a unique entity unto itself? At the virtual AAT-AD/PD meeting, held April 2 to 5, researchers reported that people with PD who carry genetic risk variants for Alzheimer’s disease were more likely to become cognitively impaired. Similarly, AD variants predicted which PD patients harbored Aβ and tau proteopathies in their brains. In people with dementia with Lewy bodies (DLB), Aβ plaques seemed to worsen cognitive decline more than tau tangles did, suggesting an etiology distinct from AD. Although the interactions between α-synuclein, Aβ, and tau still need more clarification, some suggested that therapies targeting Aβ might benefit people with Lewy body diseases.
- AD risk variants made cognitive decline likely in people with PD.
- An AD genetic risk score predicted plaques in people with PD.
- Plaques worsened tau and Lewy pathology, and cognitive decline, in people with DLB.
LBDs include PD, PD dementia (PDD), and DLB. While cognitive symptoms arise prior to movement problems in people with DLB, the opposite is true for PDD. More than 80 percent of people with PD ultimately develop dementia, but cognitive trajectories vary widely between patients. Age, male sex, severity of motor symptoms, and sleep disorders raise a person’s risk of PDD, and CSF biomarkers of AD, as well as ApoE4 genotype, have been associated with cognitive decline in PD (Tropea et al., 2018; Feb 2020 news).
Might other AD variants also influence cognitive worsening in PD? At AD/PD, Thomas Tropea of the University of Pennsylvania in Philadelphia addressed this question using genetic and longitudinal cognitive data from 305 participants in UPenn’s ongoing PD cohort. The volunteers had PD but were dementia-free when they enrolled. They took the Mattis Dementia Rating Scale-2—a cognitive composite—every year for an average of 4.4 years and up to 12 years. Tropea, who is part of Alice Chen-Plotkin’s group at UPenn, screened the participants for 22 genetic risk variants, including 20 confirmed and two candidate variants from AD GWAS (Mar 2019 news).
By now, half of the participants have dementia, and Tropea reported that certain gene variants correlated with their rate of decline on the DRS-2. While ApoE4 had the strongest effect, AD risk variants in BIN1, NYAP1, PICALM, FERMT2, SLC24A4, and ADAM10 also associated with a faster downturn. Only PICALM and ApoE4 associated with decline on all five cognitive domains included in the DRS-2; other variants correlated only with particular components. Tropea next wants to investigate the combined effects of these AD risk variants in predicting cognitive decline in people with PD.
Do AD genes also flag AD neuropathology in people with PD? Also at AAT-AD/PD, David Dai in Chen-Plotkin’s group drew on genetic and neuropathological data from 208 people who died with an LBD—55 with PD, 108 with PDD, 45 with DLB. Based on their burden of Aβ plaques and tau tangles at autopsy, Dai ranked the degree of AD neuropathology for each case as none, low, intermediate, or high. A third of the cohort had some degree of these proteopathies. Dai then fed genotypes at 20 AD risk loci from 127 of the patients into an algorithm that returned a combination of four factors—ApoE4, BIN1, and SORL1 genotypes, and age at onset—as best correlated. Dai combined these four into a risk score, which predicted the presence of plaques and tangles in a test cohort of 81 other cases. Breaking up risk scores into quintiles, Dai found that cases with risk scores in the highest quintile were four times more likely to have Aβ plaques and tangles than those in the lowest two quintiles.
All three genetic variants included in the score are implicated in Aβ production. SORL1 was more strongly associated with plaques and tangles in the LBD cohort, Dai reported. He plans to investigate this relationship further in larger cohorts, and to extend his studies to living PD patients with abnormal AD biomarkers.
How do interactions between Aβ, tau, and α-synuclein influence the clinical symptoms of Lewy body disease? Daniel Ferreira of the Karolinska Institute in Stockholm reported new results from a collaboration between European DLB consortia and the Mayo Clinic DLB cohorts (Jul 2019 news). Representing 11 centers and 417 participants, the consortia track biomarkers, cognition, and clinical progression in people with DLB. The European centers used CSF Aβ42 and p-tau as AD markers; Mayo, PiB and tau PET. They collaborated to generate cut-off values for each marker.

AD Markers in DLB. Nearly half of people with DLB had amyloid (A), with or without tau tangles (T). [Courtesy of Daniel Ferreira, Karolinska Institute.]
Ferreira reported that 39 percent of the trans-Atlantic cohort had normal Aβ and tau, while 32 percent had evidence of plaques, and 15 percent had both plaques and tangles. Interestingly, 13 percent were abnormal for tau but not Aβ. Ferreira noted that this could represent a type of tau accumulation distinct from the AD cascade.
Age most strongly predicted AD biomarkers: While 60 percent of the youngest DLB patients had neither amyloid nor tau pathology, only 20 percent of the oldest patients were negative for both. The proportion of people with abnormal tau, but not Aβ, held steady across age groups. Finally, in line with many prior studies, ApoE4 carriers in this cohort, too, were more likely to have plaques at a younger age than noncarriers.
How did these combinations of proteopathy influence DLB symptoms? Ferreira reported that while both Aβ and tau came with lower scores on the MMSE, Aβ influenced cognition the most. Tau did not associate with cognitive impairment independently of Aβ.
Curiously, the presence of tau pathology correlated with a lower risk for noncognitive symptoms of DLB, including parkinsonism and sleep disorders. The reason behind the apparent protective influence of tau tangles against certain DLB symptoms is unclear. Ferreira said this could likely complicate diagnosis. Strikingly, Aβ and tau did not synergize to influence cognition in DLB, as they do in Alzheimer’s. Together, the findings suggested that in people with DLB, Aβ is a stronger driver of cognitive decline than tau is.
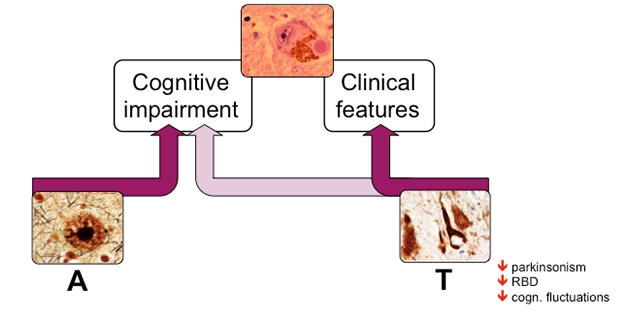
Aβ Eggs on Cognitive Decline. Lewy bodies (top) drive cognitive impairment and other clinical features of DLB. Additionally, Aβ burden (A) contributes significantly to cognitive impairment (left), while tau tangles associate with a lower frequency of other clinical features of the disease (right). [Courtesy of Daniel Ferreira, Karolinska Institute.]
Ferreira concluded that while amyloid and tau pathology are both common in people with DLB, they have distinct influences on cognition. They appear to work differently than they do in people with AD, where Aβ instigates tau pathology, which associates strongly with cognitive decline. Future studies will examine how Aβ and tau influence specific cognitive domains in people with DLB, Ferreira said.
In his talk at AAT-AD/PD, John Growdon of Massachusetts General Hospital in Charlestown made the case that Aβ-targeted therapies might be able to stem cognitive decline in people with LBD. He reviewed a decade’s worth of evidence linking Aβ to cognitive decline and markers of neurodegeneration in people with synucleinopathies. Initially, Growdon and his colleague Stephen Gomperts had reported that Aβ deposition predicted cognitive decline over a five-year follow-up in people with PD, and sped up cognitive decline in people with PDD or DLB (Gomperts et al., 2013). In 2016, they added that neurofibrillary tangles—as per tau-PET—also correlated with worse cognition in people with LBD (Sep 2016 news). More recent neuropathological findings showed that when present, Aβ deposition correlated with the severity of neurofibrillary tangles, as well as Lewy body inclusions (Shirvan et al., 2019). Finally, Growdon said that Aβ deposition correlated with cortical thinning in people with LBD, which, in turn, associated with cognitive decline (Ye et al., 2020).
Together, the studies suggest that Aβ could exacerbate both tau and α-synuclein aggregation in LBD. This, Growdon believes, is why Aβ-targeted therapies could benefit people with synucleinopathies. There was no question-and-answer period at this virtual meeting, but Growdon anticipated the main question people might ask: Anti-Aβ therapies have failed to benefit cognition in people with AD, so why would they work for LBD? In LBDs, Aβ appears to exacerbate both tau tangles and α-synuclein aggregation, either of which could lead to dementia, Growdon said. Targeting Aβ could dismantle this toxic triplet, he said.
Second, while Aβ plays the role of instigator in AD, it appears to drive progression throughout the disease process in LBD. Therefore, while ridding the brain of Aβ may be too little, too late, for people with symptomatic AD, it could slow the disease in people with LBD, Growdon reasoned.—Jessica Shugart
References
News Citations
- Toxic α-Synuclein: Egged on by ApoE4, Thwarted by ApoE2?
- Paper Alerts: Massive GWAS Studies Published
- Consortia Assemble Worldwide to Take on Lewy Body Dementia
- Tau Deepens Cognitive Trouble in Lewy Body Diseases
Paper Citations
- Tropea TF, Xie SX, Rick J, Chahine LM, Dahodwala N, Doshi J, Davatzikos C, Shaw LM, Van Deerlin V, Trojanowski JQ, Weintraub D, Chen-Plotkin AS. APOE, thought disorder, and SPARE-AD predict cognitive decline in established Parkinson's disease. Mov Disord. 2018 Feb;33(2):289-297. Epub 2017 Nov 23 PubMed.
- Gomperts SN, Locascio JJ, Rentz D, Santarlasci A, Marquie M, Johnson KA, Growdon JH. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013 Jan 1;80(1):85-91. PubMed.
- Shirvan J, Clement N, Ye R, Katz S, Schultz A, Johnson KA, Gomez-Isla T, Frosch M, Growdon JH, Gomperts SN. Neuropathologic correlates of amyloid and dopamine transporter imaging in Lewy body disease. Neurology. 2019 Jul 30;93(5):e476-e484. Epub 2019 Jun 26 PubMed.
- Ye R, Touroutoglou A, Brickhouse M, Katz S, Growdon JH, Johnson KA, Dickerson BC, Gomperts SN. Topography of cortical thinning in the Lewy body diseases. Neuroimage Clin. 2020 Jan 31;26:102196. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Penn Neurological Institute
Our studies suggest that AD genetic risk factors really play a role in whether people with PD develop dementia and the characteristic neuropathology seen in AD. This has implications for whether we might use Aβ- and tau-targeting therapies to treat what is in my mind the most disabling aspect of PD—the cognitive decline and dementia—before it's too late. In AD, the trials for these therapies have been disappointing. This could be because the targets are wrong. Or it could be because the patients are too late in disease course. At the time of PD diagnosis, people are not cognitively impaired, and the majority don't, to the best of our knowledge, have a lot of Aβ and tau pathology. This suggests to me that PD might be a really good "prodromal" population for AD-related processes. As such, there is a window, and a very at-risk population, for targeted therapies.
Make a Comment
To make a comment you must login or register.