Does Alzheimer’s Start in the Heart of the Cholinergic System?
Quick Links
Three the approved drugs for Alzheimer’s disease attempt to boost the brain’s supply of acetylcholine, a critical neurotransmitter for cognition that wanes in people with the disease. But might crumbling of the brain’s cholinergic system be one of the earliest changes in the preclinical stages of the AD cascade? According to new evidence from longitudinal studies presented at the virtual AAT-AD/PD meeting, held remotely April 2 to 5, the answer could be yes. Buoyed by advances in brain mapping and neuroimaging, researchers reported that in cognitively normal people with AD biomarkers, shrinkage of the nuclear basalis of Meynert (NbM)—an already tiny speck in the basal forebrain—predicted a rise in markers of neuroinflammation, shrinkage in the medial temporal lobe, and cognitive decline. Damage to the diminutive NbM also foreshadowed dementia in people with Parkinson’s disease. Together, the findings bestow a minuscule patch of cells deep in the brain with outsize power—or vulnerability—in neurodegenerative disease.
- In people with AD biomarkers, a shrunken nuclear basalis of Meynert predicted microglial neurotoxicity and inflammation.
- This preceded memory loss, and atrophy in the medial temporal lobe.
- In people with Parkinson’s, a small nuclear basalis of Meynert predicted cognitive decline.
Cholinergic neurons reside in distinct clusters, called nuclei, within the basal forebrain and within the brainstem. Projections from these nuclei supply acetylcholine (ACh) to different regions of the brain. The NbM, aka Ch4, is the major cholinergic supplier of the cortex, and is more strongly tied to cognition than any of the other nuclei. Scientists visualize cholinergic neurons in postmortem samples by immunostaining for the enzymes choline acetyltransferase (ChAT) and acetylcholine esterase (AChE). In recent years, advanced imaging and stereology have created precise anatomical maps of the NbM, facilitating longitudinal measurements of its size via structural MRI (Zaborsky et al., 2008; Kilimann et al., 2014).
Applying these techniques in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort, researchers led by Taylor Schmitz and Nathan Spreng previously tied smaller NbM volume to subsequent shrinkage of the entorhinal cortex (EC) and to cognitive decline (Nov 2016 news). They placed degeneration in the NbM upstream of atrophy in the EC, to some controversy in the field.
At AD/PD, Sara Fernández-Cabello of the University of Salzburg in Austria presented follow-up ADNI data, which included more participants, more timepoints, and more powerful statistical analyses connecting the dots between degeneration of the NbM and other regions of the brain. Drawing on more than 835 participants in ADNI who had CSF measurements and structural MRI scans over two years, Fernández-Cabello reported that small NbM size at baseline predicted subsequent shrinkage of the EC, but not vice versa. In turn, a smaller EC predicted atrophy in temporal cortical regions including the entorhinal, perirhinal, parahippocampal, and fusiform cortices and temporal poles, as well as in some clusters within the insular and posterior parietal cortices.
Importantly, this degenerative chain of events took place only in people with an abnormal CSF ratio of p-tau to Aβ42, regardless of their cognitive status. In other words, they represent steps of AD-specific pathogenesis. The findings were published March 1 in Brain (Fernandez-Cabello et al., 2020).
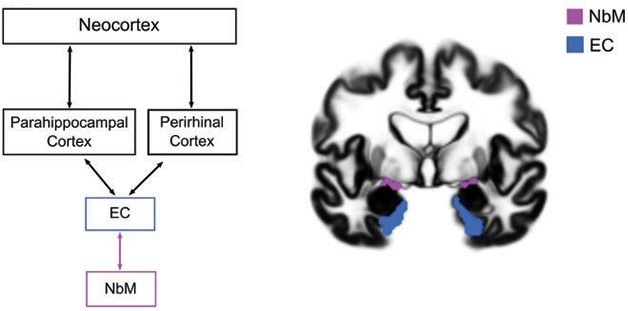
NbM Upstream? An updated version of the AD neurodegenerative staging scheme places the NbM upstream of the EC. [Courtesy of Fernandez-Cabello et al., Brain, 2020.]
At AAT-AD/PD, Fernández-Cabello showed additional, unpublished findings from the same ADNI cohorts, linking degeneration in the NbM with different types of cognitive dysfunction. The researchers found that a smaller NbM at baseline predicted worsening scores on tests of memory and executive function. They then pieced together which change mediated which other change. They found that, among people with abnormal AD biomarkers, the memory loss triggered by NbM degeneration was mediated via atrophy in temporoparietal and medial regions. In all, the findings suggest a pathological chain of events unfolding uniquely in people with AD biomarkers, in which shrinkage of the NbM leads to subsequent degeneration of temporoparietal regions, ultimately manifesting in memory loss.
At AD/PD, Taylor Schmitz of Western University in Ontario described another consequence of NbM degeneration in people with AD: the transformation of microglia from helper to harmer. Numerous studies have described how microglia transition from a homeostatic, or neuroprotective, state, to an inflammatory, neurotoxic state in people with AD and in animal models of amyloidosis and tauopathy (Jan 2017 news; Sep 2017 news; May 2019 news). Schmitz blames a floundering cholinergic system, citing previous findings that cholinergic stimulation not only quells inflammation in the peripheral nervous system, but also calms microglia in the CNS via stimulation of microglial alpha 7 nicotinic acetylcholinergic receptors (nAChR) (Wang et al., 2003; Shytle et al., 2004; De Simone et al., 2005) Does a dwindling cholinergic supply unleash toxic microglial inflammation?
To test this idea, Schmitz used longitudinal imaging and biomarker data from ADNI. He partitioned 268 cognitively normal volunteers into “neurotypical aging” or “preclinical AD” subgroups based on their CSF p-tau/Aβ42 ratio. He then tracked NbM volume on MRI over two years, finding that people with abnormal biomarkers not only had a smaller NbM at baseline, but also lost more NbM volume than people in the neurotypical aging group did. The researchers also tracked soluble CSF TREM2, as its rise coincides with the emergence of tau pathology in AD and is thought to signify a transition of microglia into a neurotoxic state (Jan 2016 news).
Schmitz and colleagues found that typical aging and preclinical AD groups started out with similar concentrations of sTREM2. Over two years, sTREM2 rose slightly in both groups, with the latter trending toward more elevation than the former. NbM shrinkage and rising sTREM2 concentration were strongly correlated, but only in the preclinical AD group.
Mirroring the CSF sTREM2 finding, plasma complement C3 also crept up along with NbM degeneration in the preclinical AD group. Together, the findings support the idea that in preclinical AD, NbM degeneration correlates with a rise in neuroinflammation. The results were published February 26 in the Journal of Neuroscience (Schmitz et al., 2020).
Schmitz acknowledged that he cannot formally prove that NbM degeneration causes neuroinflammation in preclinical AD. Nor does he know what drives C3 to increase in the blood in preclinical AD. This could derive from signals in the brain, for example cytokines that inflame the vascular endothelium of the blood brain barrier. Alternatively, a loss of cholinergic output from the vagus nerve could lead to activation of macrophages throughout the body. It was via this peripheral pathway that the anti-inflammatory effects of acetylcholine were first established, Schmitz noted.
Another limitation of the ADNI volume data is its lack of specificity for cholinergic neurons, Schmitz said. Theoretically, dying non-cholinergic cells in close proximity to cholinergic neurons could contribute to shrinkage in the NbM. Schmitz and colleagues are developing a PET tracer for vesicular acetylcholine transferase, a protein expressed in presynaptic terminals of cholinergic neurons. Mouse experiments show a cholinergic system all aglow, Schmitz said, and a pilot study testing the tracer in humans is underway.
Researchers led by Zhude Tu at Washington University in St. Louis have developed another VAChT PET tracer. In his abstract at AD/PD, Tu reported that the first human study of that tracer showed cholinergic synapses in multiple regions of the brain decrease with age.
Yet another approach to gauge if the cholinergic system is healthy is to measure the integrity of the axonal projections that spring forth from the basal forebrain. At AD/PD, Milan Nemy of the Czech Technical University in Prague described how he used diffusion tensor imaging to trace cholinergic circuitry in 262 healthy volunteers between 39 to 77 years of age (Nemy et al., 2020). Nemy also took stock of the NbM volume and white-matter hypointensities, a proxy for small-vessel disease. Nemy reported that the cholinergic circuitry eroded with age; this erosion correlated more strongly with poorer performance on memory and attention tests than did white-matter hypointensities or NbM volume, suggesting that weakening cholinergic connections could manifest as cognitive decline.
Schmitz considers the white-matter tractography data compelling, in that it likely captured at least some of the basal forebrain projectome. Still, he believes, tractography comes with the same limitation as measuring NbM volume: It’s not specific to cell type. The farther one moves from the basal forebrain, the more difficult it is to definitively link axonal projections to cholinergic neurons, Schmitz said, suggesting that, ultimately, PET tracers will most clearly delineate the cholinergic system.
A puny NbM also foretold of cognitive trouble in people with Parkinson’s. In her presentation at AAT-AD/PD, Joana Pereira of the Karolinska Institute in Stockholm described findings from the Swedish BioFinder study, which tracked NbM volume and cognition over an average of four years in a cohort of 106 nondemented people with PD, 20 people with Parkinson’s Disease Dementia (PDD), and 42 healthy controls (Pereira et al., 2020).
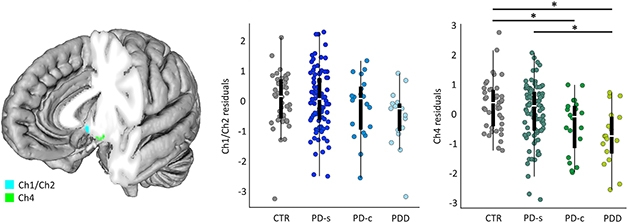
Forebrain Foretells Dementia in PD? The nucleus basalis of Meynert (Ch4, green) is smaller in people with PDD or PD who later converted to dementia (PD-c) than in people who never developed dementia (CTR, PD-s). [Courtesy of Pereira et al., Neurobiology of Disease, 2020.]
At baseline, people with PDD, or those with PD whose cognition would later decline, had smaller NbM volumes than did healthy controls or people with PD who did not. NbM atrophy over time associated with waning global cognition and verbal fluency, as well as diagnosis of dementia. The medial septum/diagonal band—another cholinergic cluster in the basal forebrain—also shrank more over time in people with PDD or in those who later developed dementia. Together, the findings suggested that degeneration of cholinergic basal forebrain structures might influence the clinical heterogeneity of PD.
“There are now several studies showing that the NbM could be a non-invasive marker of cholinergic dysfunction and dementia in AD and PD,” Pereira wrote to Alzforum. Despite substantial overlap in NbM volume between groups, Pereira proposed that NbM atrophy could be used to identify people at highest risk for cognitive decline, or even as a biomarker in clinical trials aimed at preventing dementia.—Jessica Shugart
References
News Citations
- Are Cholinergic Neurons “Patient Zero” of Alzheimer’s Disease?
- Microglia Give Astrocytes License to Kill
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- When It Comes to Alzheimer’s Disease, Do Human Microglia Even Give a DAM?
- TREM2 Goes Up in Spinal Fluid in Early Alzheimer’s
Paper Citations
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008 Sep 1;42(3):1127-41. Epub 2008 Jun 7 PubMed.
- Kilimann I, Grothe M, Heinsen H, Alho EJ, Grinberg L, Amaro E Jr, Dos Santos GA, da Silva RE, Mitchell AJ, Frisoni GB, Bokde AL, Fellgiebel A, Filippi M, Hampel H, Klöppel S, Teipel SJ. Subregional basal forebrain atrophy in Alzheimer's disease: a multicenter study. J Alzheimers Dis. 2014;40(3):687-700. PubMed.
- Fernández-Cabello S, Kronbichler M, Van Dijk KR, Goodman JA, Spreng RN, Schmitz TW, Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain volume reliably predicts the cortical spread of Alzheimer's degeneration. Brain. 2020 Mar 1;143(3):993-1009. PubMed.
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003 Jan 23;421(6921):384-8. PubMed.
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004 Apr;89(2):337-43. PubMed.
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005 Jan 25;2(1):4. PubMed.
- Schmitz TW, Soreq H, Poirier J, Spreng RN. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer's Disease. J Neurosci. 2020 Feb 26;40(9):1931-1942. Epub 2020 Jan 8 PubMed.
- Nemy M, Cedres N, Grothe MJ, Muehlboeck JS, Lindberg O, Nedelska Z, Stepankova O, Vyslouzilova L, Eriksdotter M, Barroso J, Teipel S, Westman E, Ferreira D. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020 May 1;211:116607. Epub 2020 Feb 6 PubMed.
- Pereira JB, Hall S, Jalakas M, Grothe MJ, Strandberg O, Stomrud E, Westman E, van Westen D, Hansson O. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson's disease. Neurobiol Dis. 2020 Jun;139:104831. Epub 2020 Mar 5 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Fernández-Cabello S, Kronbichler M, Van Dijk KR, Goodman JA, Spreng RN, Schmitz TW, Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain volume reliably predicts the cortical spread of Alzheimer's degeneration. Brain. 2020 Mar 1;143(3):993-1009. PubMed.
- Schmitz TW, Soreq H, Poirier J, Spreng RN. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer's Disease. J Neurosci. 2020 Feb 26;40(9):1931-1942. Epub 2020 Jan 8 PubMed.
- Pereira JB, Hall S, Jalakas M, Grothe MJ, Strandberg O, Stomrud E, Westman E, van Westen D, Hansson O. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson's disease. Neurobiol Dis. 2020 Jun;139:104831. Epub 2020 Mar 5 PubMed.
- Nemy M, Cedres N, Grothe MJ, Muehlboeck JS, Lindberg O, Nedelska Z, Stepankova O, Vyslouzilova L, Eriksdotter M, Barroso J, Teipel S, Westman E, Ferreira D. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020 May 1;211:116607. Epub 2020 Feb 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Amsterdam UMC
The recent findings of Schmitz and colleagues on the loss of cholinergic integrity in the brain are consistent with those of Jan Willem van Dalen from our group. Based on diffusion tensor imaging and tractography in 87 memory clinic patients, van Dalen reported that degradation of the projections from the nucleus basalis of Meynert is associated with specific clinical symptoms as well as a trend for higher mortality.
References:
van Dalen JW, Caan MW, van Gool WA, Richard E. Neuropsychiatric symptoms of cholinergic deficiency occur with degradation of the projections from the nucleus basalis of Meynert. Brain Imaging Behav. 2017 Dec;11(6):1707-1719. PubMed.
Make a Comment
To make a comment you must login or register.