NfL: Useful in Differential Diagnosis?
Quick Links
In a comprehensive meta-analysis, scientists led by Claire Bridel, VU University Medical Center, Amsterdam, compared levels of neurofilament light in the cerebrospinal fluid of healthy controls and patients covering a spectrum of neurological disorders. In the June 17 JAMA Neurology, they report that while NfL levels are elevated in most neurodegenerative diseases, they are particularly high in frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and the cognitive impairment that accompanies infection with HIV. The findings suggest CSF NfL could aid in the differential diagnosis of these and other disorders.
- Meta-analysis compares CSF NfL across 32 neurological disorders.
- The highest levels occur in FTD, ALS, HIV infection, and HD.
- NfL may distinguish FTD from other dementias, and PD from other parkinsonisms.
“It’s a landmark study on the potential diagnostic use of neurofilament light,” said co-author Marcel Verbeek, Radboud University, Nijmegen, The Netherlands. This systematic review brings all the data from individual case-control studies of NfL in disparate diseases together in one place, putting them in a bigger context, he said.
First author Bridel and colleagues searched PubMed for “cerebrospinal fluid” and “neurofilament light,” limiting their search to publications between 2006 and 2016. They included studies in healthy control groups, people with cognitive complaints, and patients with psychiatric diseases that affect the central nervous system. To minimize variability due to different NfL assays, they analyzed only the studies that used a commercially available ELISA assay from Uman Diagnostics. Forty-seven studies met all these criteria. The authors obtained the individual level data from each, making sure to glean information on the age, sex, and diagnosis of each individual, as well as the treatment status where applicable.
In the end, they reanalyzed raw data from 10,012 people, comprising 32 diagnostic categories. These included inflammatory diseases of the CNS, parkinsonian disorders, dementias, and predementia states.
Across diagnoses, CSF NfL was higher than in healthy controls. This was especially true for impairment in HIV (iHIV), FTD/amyotrophic lateral sclerosis, ALS itself, and Huntington’s disease, with levels averaging 21-, 10.5-, 7.6-, and 5.8-fold higher, respectively, than in controls (see image below). By comparison, AD and PD NfL levels were 1.86 and 1.08 higher than controls, respectively. The study confirmed that NfL is elevated across the board, and because values overlap among diseases, it has little value as a specific marker for any of them.

NfL Spectrum. A comparison of CSF NfL levels (log transformed) for healthy controls (top), all the way to iHIV (bottom). Average FTD levels are higher than in AD. Dots represent outliers. [Courtesy of Bridel et al., 2019.]
What about differential diagnosis? Because the average NfL levels were so much higher in FTD, it could be used to differentiate it from Alzheimer’s, vascular dementia, and dementia with Lewy bodies (DLB), the authors wrote. Yolande Pijnenburg, also at VUMC, said that in its early stages FTD can be hard to distinguish clinically from other disorders as well, such as bipolar disorder and depression, and NfL could help. “It has been a struggle to find a suitable biomarker for FTD, probably due to the multiple underlying pathologies of this disease,” she wrote.
In Parkinson’s disease (PD), PD dementia (PDD), and DLB, NfL levels overlapped, but they were all significantly below those seen in multiple system atrophy (MSA), corticobasal syndrome (CBS), or progressive supranuclear palsy (PSP), suggesting that CSF NfL could help differentiate PD from these atypical parkinsonian disorders. Historically, this has been difficult to do, especially at early stages of PD.
As has been reported before, Bridel and colleagues found that CSF NfL rose with age in healthy controls (see image below). This could reflect age-related neuron loss, ongoing preclinical neurodegenerative disease, or reduced CSF clearance with age, said senior author Charlotte Teunissen, VUMC. The authors found NfL rose in people with most dementias as they aged as well, with the exception of FTD and ALS. In contrast, NfL levels held steady as patients with neuroinflammatory disorders got older, and even decreased with age in untreated relapsing-remitting multiple sclerosis (uRRMS). Perhaps early and massive tissue loss in aggressive neurodegenerative or neuroinflammatory disorders masks age-related effects, suggested Teunissen. In other words, fewer neurons are around in later years to degenerate and contribute to the pool of NfL in the CSF.
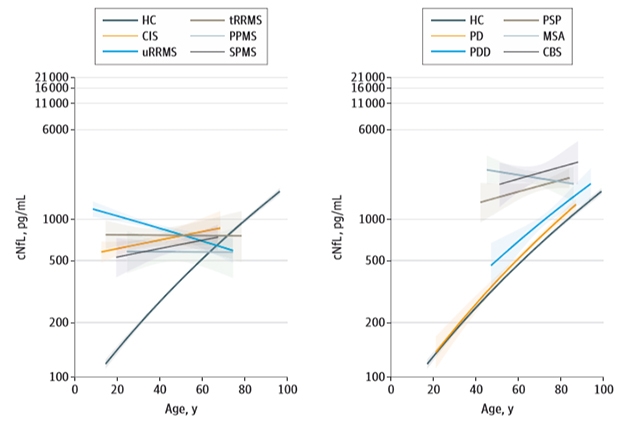
Lifetime Changes. As healthy controls age, NfL rises in the CSF (black line). The same happens in most dementias except FTD (left) and in some parkinsonian disorders ones (right). [Courtesy of Bridel et al., 2019 JAMA Neurol.]
If CSF NfL does prove useful as a differential diagnostic marker, then plasma NfL might also. Previous studies have suggested a high concordance between the two (Jun 2016 news; Apr 2016 conference news). More recently, researchers led by Michelle Mielke, Mayo Clinic, Rochester, Minnesota, reported how changes in NfL over time tracked with other markers of neurodegeneration among 79 normal older adults taking part in the Mayo Clinic Study of Aging. An increase in both CSF and plasma NfL over 15 to 30 months correlated with increased amyloid PET and worse global cognition and attention. The findings, published in the June 10 Neurology, are in line with both DIAN and ADNI cohort studies (Jan 2019 news; May 2019 news). Stephanie Schultz, a graduate student in the lab of Tammie Benzinger at Washington University in St. Louis, said the Mayo data will help translate the value of NfL to more heterogeneous populations. Their community-based study does not exclude people who have vascular problems or other comorbidities, she noted.—Gwyneth Dickey Zakaib
References
News Citations
Further Reading
Papers
- Zhao Y, Xin Y, Meng S, He Z, Hu W. Neurofilament light chain protein in neurodegenerative dementia: A systematic review and network meta-analysis. Neurosci Biobehav Rev. 2019 Jul;102:123-138. Epub 2019 Apr 24 PubMed.
- Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, Simonsen AH, Verbeek MM, Rosa-Neto P, Slot RE, Tainta M, Izaguirre A, Reijs BL, Farotti L, Tsolaki M, Vandenbergue R, Freund-Levi Y, Verhey FR, Clarimón J, Fortea J, Frolich L, Santana I, Molinuevo JL, Lehmann S, Visser PJ, Teunissen CE, Zetterberg H, Blennow K. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer's disease continuum in the BIOMARKAPD study. Alzheimers Dement. 2019 Jun;15(6):742-753. Epub 2019 Apr 6 PubMed.
- Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019 Aug;90(8):870-881. Epub 2019 Apr 9 PubMed.
Primary Papers
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFL Group, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L, Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu CH, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J, Piehl F, Pijnenburg YA, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C, Wild EJ. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019 Jun 17; PubMed.
- Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, Machulda MM, Kremers WK, Knopman DS, Jack C Jr, Petersen RC, Kern S. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019 Jul 16;93(3):e252-e260. Epub 2019 Jun 10 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Hospital de Sant Pau
I found this study particularly interesting because NfL in CSF (and probably in plasma) may be incorporated soon in clinical routine to the current biomarker panel. The strength of NfL is that, since it is only expressed in the nervous system, it can capture the global neuronal/axonal damage and translate it into one numerical value.
The interest of the study relies on the large sample size and diversity of diagnoses, and although (as the authors mention) there is no reference method for NfL, the fact that the authors only included those studies that used the assay from Uman Diagnostics.
It was quite surprising to see that the HIV group had the highest levels, followed by FTD/ALS (already known from other studies). This may be explained by the axonal damage in subcortical regions in these disorders but could also mean that neuroinflammation (which is particularly present in HIV) could be driving this increase.
The differences in sex in CSF NfL levels (males higher levels than females) were also intriguing (also observed in a large recent multicenter study by Lleó et al. 2019), because most biomarkers do not show detectable sex differences. Of note, this difference has not been observed in blood samples. The reasons for these differences remain to be investigated and therefore it will be important to take into account sex for defining specific cut-offs for some diseases.
The main potential clinical use of NfL however, relies not on its diagnostic potential (limited by the overlap seen between most of the disorders) but on its excellent correlation with progression of the disease in many conditions. In some diseases, such as AD, this offers the possibility of having diagnostic (Aβ42 and 40 and t-tau, p-tau) and prognostic markers (NfL) with one single procedure.
The value of NfL in CSF could be reinforced by the possibility of measuring NfL in blood samples, which are more accessible than CSF, in follow-up visits, precluding the need of repeating the lumbar puncture.
This study also suggest that NfL levels in CSF could be useful in certain particular scenarios, for example to in the differential diagnosis between AD and FTD (alone or in combination with others CSF markers, such sAPPβ, which is low in FTD and normal in AD (Alcolea et al., 2017) and between PD and atypical parkinsonisms.
Finally, I would like to mention how studies with CSF markers have shown distinct profiles between AD and FTD, with AD-neurodegeneration closely associated to tau and FTD-neurodegeneration more associated to NfL. This clearly shows that tau and NfL provide information on neurodegeneration that is, at least in part, different.
References:
Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, Simonsen AH, Verbeek MM, Rosa-Neto P, Slot RE, Tainta M, Izaguirre A, Reijs BL, Farotti L, Tsolaki M, Vandenbergue R, Freund-Levi Y, Verhey FR, Clarimón J, Fortea J, Frolich L, Santana I, Molinuevo JL, Lehmann S, Visser PJ, Teunissen CE, Zetterberg H, Blennow K. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer's disease continuum in the BIOMARKAPD study. Alzheimers Dement. 2019 Jun;15(6):742-753. Epub 2019 Apr 6 PubMed.
Alcolea D, Vilaplana E, Suárez-Calvet M, Illán-Gala I, Blesa R, Clarimón J, Lladó A, Sánchez-Valle R, Molinuevo JL, García-Ribas G, Compta Y, Martí MJ, Piñol-Ripoll G, Amer-Ferrer G, Noguera A, García-Martín A, Fortea J, Lleó A. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology. 2017 Jul 11;89(2):178-188. Epub 2017 Jun 7 PubMed.
Make a Comment
To make a comment you must login or register.