Electrode Detects Aβ Aggregates in Alzheimer’s Plasma
Quick Links
In the April 17 Science Advances, researchers proffer a new technique for detecting plasma Aβ. Scientists led by Kyo Seon Hwang at Kyung Hee University, Seoul, and YoungSoo Kim at Yonsei University, Incheon, both in the Republic of Korea, detail a protocol to dissociate Aβ multimers into their component monomers, then measure them with a microfluidic electrode. Their data suggest that people with Alzheimer’s disease have more Aβ oligomers in their blood than monomers. This higher ratio distinguished clinically diagnosed patients with greater than 90 percent specificity and sensitivity. Most other studies report that plasma Aβ42 concentrations fall as AD progresses and more of the peptide gets stuck in the brain.
- A new technique breaks down plasma Aβ into monomers and detects them with an antibody.
- AD patients have a higher number of plasma Aβ monomers than controls.
- The method distinguishes AD from healthy people with >90 percent sensitivity and selectivity.
Some scientists who spoke with Alzforum were skeptical, pointing out that the sample sizes were small and heterogeneous. Charlotte Teunissen and Inge Verberk, Amsterdam University Medical Centers, VU University, both questioned whether Aβ oligomers even exist in the blood. “We have a really hard time detecting them in cerebrospinal fluid,” Teunissen said. Nevertheless, they found merits in the study. “It fits with the idea that amyloid is detectable in the blood and relates to AD,” said Teunissen. Verberk thinks that dissociating Aβ complexes into monomers could be informative. This pretreatment method could be validated using other amyloid blood assays, she said.

Breaking It Down. Plasma samples are halved, with one portion treated to dissociate Aβ multimers into monomers. Both are tested with an Aβ-specific microelectrode. The ratio reveals the relative concentration of aggregates in the original sample. [Courtesy of Science Advances/AAAS.]
Scientists are making rapid progress toward a blood test for Aβ, with bead-based Simoa assays and mass spectrometry leading the pack (Jul 2017 news; Feb 2018 news; Aug 2018 news; Feb 2019 news).
Hwang and colleagues previously introduced another way to detect Aβ using an interdigitated microelectrode (IME) system (Yoo et al., 2017). In this setup, microfluidic channels in a dimethicone layer sit atop a silicon sensor chip with platinum electrodes printed on it. The researchers coat this lower sensor with an Aβ antibody, in this case 6E10. When a plasma sample enters the microfluidic channels, any Aβ that sticks to the antibody causes a voltage across the electrodes to drop. This impedance is then used to calculate how much Aβ is in the sample. The limit of detection is 0.1 picograms/mL.
In the current study, YoungSoo Kim and Hye Yun Kim added a pre-analytical step. They used a small molecule called EPPS [4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid], which, according to authors, breaks down aggregated Aβ in plasma (Kim et al., 2015). They reasoned that aggregates would be difficult to detect because epitopes may be inaccessible to the antibody. A previous study reported that blood from AD patients has more oligomers of Aβ (Zhang et al., 2014).
Kim and colleagues, including joint first authors Jee Hoon Roh, University of Ulsan College of Medicine, and Yong Kyung Yoo, Kwangwoon University, both in Seoul, tested their IME/EPPS system on blood samples from AD patients and controls from two institutions in Korea—the Asan Medical Center (AMC) and Korea Institute of Radiological & Medical Sciences (KIRAMS). Sixty-one patients were clinically diagnosed with AD and 45 age-matched participants were cognitively normal. Plasma samples from each were divided in half and one part was treated with EPPS. Both aliquots were tested with the IME chip. The concentration of Aβ in the untreated sample served as an internal standard, intended to minimize inter- and intra-sample variation. The authors reported what they called a CLASS (comparing levels of Aβ by self-standard) ratio.
That ratio distinguished AD patients from controls with 93 percent sensitivity and 97 percent specificity (see image below). The researchers calculated cutoff CLASS ratios for AD of 1.3 and 1.2 for the AMC and KIRAMS cohorts, respectively. The ratio correlated with both global standardized uptake value ratios (SUVR) in amyloid-PET scans and with the Korean version of the mini-mental state examination (MMSE). This suggested to the authors that the ratio correlates with disease stage. Many studies have asserted that Aβ levels change little once disease begins.
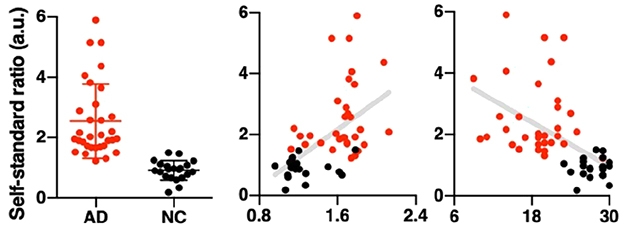
Clean Separation? The CLASS ratio distinguishes AD (red) from control subjects (black) with some overlap (left) and modestly correlates with both SUVR from amyloid PET scans (middle) and cognition as measured by MMSE (right). [Courtesy of Science Advances/AAAS.]
Despite having amyloid PET data, that authors did not use it as a diagnostic. “The availability of amyloid PET is a strength of the study but it wasn’t fully utilized,” wrote Michelle Mielke, Mayo Clinic, Rochester, Minnesota. Instead of comparing the blood biomarker with amyloid status, the researchers used the more heterogeneous clinical diagnosis, she said. Some controls were amyloid-positive and some patients appeared to be amyloid-negative (see image above). A handful of patients who were diagnosed with AD but had low SUVRs also had high CLASS ratios, raising questions about the specificity of the plasma test.
For his part, Hilal Lashuel, Ecole polytechnique fédérale de Lausanne (EPFL), Switzerland, questioned how EPPS interacts with fibrils and oligomers to disassociate them into Aβ monomers. He also wondered how Aβ then stays in monomeric form. “More data on the detailed mechanism and kinetics of EPPS-mediated disaggregation would be helpful to assess and validate their proposed mechanism of action,” he said.
Much more work is required to demonstrate whether this CLASS ratio can distinguish between the various stages of AD, wrote Colin Masters, University of Melbourne, Australia, to Alzforum (see comment below). With such a small sample size, the results seem preliminary, he contended. He noted that this electrode assay, and another assay called Immunomagnetic Reduction–Superconducting Quantum Interference (MagQu), which measures Aβ42, are the only two that find increased Aβ in the plasma in AD (Yang et al., 2017). “This is a paradox that remains to be resolved,” he said. “The answer may contain some important insights into AD pathogenesis.”
Teunissen noted that both the IME and MagQu assays only use one antibody, and that having separate capture and detection antibodies greatly increases specificity. She expects mass spectrometry would have revealed any increase in oligomers or aggregates, since by design it breaks proteins apart, but there are no such reports.
The authors wrote that they would be interested to try different detection antibodies, particularly any that specifically bind monomers or that even distinguish between Aβ40 and Aβ42.—Gwyneth Dickey Zakaib
References
News Citations
- Finally, a Blood Test for Alzheimer’s?
- Closing in on a Blood Test for Alzheimer’s?
- With Sudden Progress, Blood Aβ Rivals PET at Detecting Amyloid
- Blood Test Granted Breakthrough Status, To Be Tested in Trial
Antibody Citations
Paper Citations
- Yoo YK, Kim J, Kim G, Kim YS, Kim HY, Lee S, Cho WW, Kim S, Lee SM, Lee BC, Lee JH, Hwang KS. A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer's disease. Sci Rep. 2017 Aug 21;7(1):8882. PubMed.
- Kim HY, Kim HV, Jo S, Lee CJ, Choi SY, Kim DJ, Kim Y. EPPS rescues hippocampus-dependent cognitive deficits in APP/PS1 mice by disaggregation of amyloid-β oligomers and plaques. Nat Commun. 2015 Dec 8;6:8997. PubMed.
- Zhang J, Peng M, Jia J. Plasma Amyloid-β Oligomers and Soluble Tumor Necrosis Factor Receptors as Potential Biomarkers of AD. Curr Alzheimer Res. 2014 Mar 16; PubMed.
- Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of Plasma Biomarkers Using Immunomagnetic Reduction: A Promising Method for the Early Diagnosis of Alzheimer's Disease. Neurol Ther. 2017 Jul;6(Suppl 1):37-56. Epub 2017 Jul 21 PubMed.
Further Reading
Papers
- Rózga M, Bittner T, Batrla R, Karl J. Preanalytical sample handling recommendations for Alzheimer's disease plasma biomarkers. Alzheimers Dement (Amst). 2019 Dec;11:291-300. Epub 2019 Apr 2 PubMed.
- Li WW, Shen YY, Tian DY, Bu XL, Zeng F, Liu YH, Chen Y, Yao XQ, Li HY, Chen DW, Zhou FY, Yang H, Li QM, Bao WQ, Guan YH, Zhou HD, Jin RB, Wang YJ. Brain Amyloid-β Deposition and Blood Biomarkers in Patients with Clinically Diagnosed Alzheimer's Disease. J Alzheimers Dis. 2019;69(1):169-178. PubMed.
Primary Papers
- Kim Y, Yoo YK, Kim HY, Roh JH, Kim J, Baek S, Lee JC, Kim HJ, Chae MS, Jeong D, Park D, Lee S, Jang H, Kim K, Lee JH, Byun BH, Park SY, Ha JH, Lee KC, Cho WW, Kim JS, Koh JY, Lim SM, Hwang KS. Comparative analyses of plasma amyloid-β levels in heterogeneous and monomerized states by interdigitated microelectrode sensor system. Sci Adv. 2019 Apr;5(4):eaav1388. Epub 2019 Apr 17 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Melbourne
The authors have developed a microfluidics-based, solid-state impedance measure (interdigitated microelectrode [IME] sensor system) of monoclonal antibody 6E10-Aβ interactions which they claim can measure plasma Aβ levels on a scale of 0.1 pg/ml. The results appear very preliminary, with only small numbers of test subjects from two Korean sites (n=53 at each) and roughly equal numbers of subjects with normal cognition (NC) and persons with AD dementia. One site used florbetaben-PET and the other used an unvalidated tracer FC119S for screening of participants.
The plasma assay depends on the effects of adding the small molecule hydroxyethyl-piperazinepropanesulfonic acid (EPPS), which is intended to dissociate Aβ complexes and aggregates, thus allowing an assay for total plasma Aβ. The results show an increased signal in the AD group, but the sample numbers are too small to allow any conclusions on performance of detecting Aβ-positive subjects in the CN groups. Much more work is required to demonstrate specificity for the various stages of AD throughout its 30-year natural history of onset and progression.
This assay therefore joins with the Taiwanese MagQu test (Immuno-Magnetic Reduction–Superconducting Quantum Interference [IMR-SQUID] technique, Yang et al., 2017) in that both are showing increased signals in AD, whereas most other high-performance assays (mass spectrometric and immunoassays) are driven by a decrease in the Aβ42 signal. This is a paradox which remains to be resolved. The answer may contain some important insights into AD pathogenesis.
References:
Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of Plasma Biomarkers Using Immunomagnetic Reduction: A Promising Method for the Early Diagnosis of Alzheimer's Disease. Neurol Ther. 2017 Jul;6(Suppl 1):37-56. Epub 2017 Jul 21 PubMed.
Make a Comment
To make a comment you must login or register.