Too Phatal: How Microglia, Astrocytes Snuff Out Dying Neurons
Quick Links
Conventional wisdom holds that microglia are the main phagocytes of the brain, mopping up dead cells and debris. The reality is more complicated, according to scientists led by Jaime Grutzendler at Yale School of Medicine in New Haven, Connecticut. In today’s Science Advances, they show how microglia and astrocytes work as a team to clear dying neurons. The authors triggered apoptosis in single cortical neurons in the brains of living mice, then watched what happened to these cells over the next several days. Nearby microglia moved in to engulf the cell body and its proximal dendrites. Meanwhile, nearby astrocytes gobbled up the degenerating dendritic arbor. The two types of phagocyte each stayed in their lane, with sharp boundaries between their territories. Importantly, the efficiency of this cleanup crew waned as mice aged, with disposal of dead neurons taking twice as long in elderly animals.
- 2Phatal method lets scientists trigger apoptosis in a single neuron, then watch it die.
- One microglia devours its soma while astrocytes absorb the dendritic arbor.
- Both types of phagocyte need the receptor Mertk for this.
“This elegant study reveals a coordinated and finely orchestrated action of both microglia and astrocytes during cell death,” Marco Colonna and Simone Brioschi at Washington University in St. Louis wrote to Alzforum (full comment below). “It will be interesting to see how this technique will be exploited to study microglia- versus astrocyte-mediated phagocytosis in pathological settings.”

Division of Labor. In mouse brain, microglia (green) clean up the soma and proximal dendrites of a dying neuron (white); astrocytes (red) tidy up distant dendrites. Where green meets red, there is a boundary. [Courtesy of Damisah et al., Science Advances.]
Although microglia have been regarded as the main phagocytic cells in the brain, astrocytes have been reported to gobble up apoptotic cells in developing brain and after ischemic damage, and to eat Aβ in vitro (Mar 2003 news; Iram et al., 2016; Morizawa et al., 2017). It was unknown under what conditions each cell type assumed cleanup duties, and whether they acted independently.
To investigate this, Grutzendler and colleagues deployed the technique two-photon apoptotic targeted ablation (2Phatal), which they had previously devised to observe the clearance of single cells over time in situ. In this procedure, the researchers apply a DNA-binding dye to the cortices of live mice through a cranial window. The dye penetrates up to 300 microns deep, labeling all nuclei. The scientists then train a two-photon laser on a single neuron and photobleach the dye with a pulse of light. This generates free radicals that damage DNA and trigger apoptosis in the targeted cell. The crime unfolds within a couple of hours, and causes no collateral damage to surrounding cells (Hill et al., 2017).
The 2Phatal technique enables researchers to induce apoptosis in the living brain with spatial and temporal precision, and follow the process dynamically, Grutzendler noted. “I’m not aware of any other technique that can look at the kinetics of cell death and engulfment,” he told Alzforum.
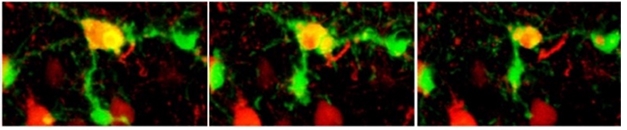
Winner Take All. Three nearby microglia (green) initially contact (left) a dying neuron (yellow). The middle one prevails and moves in (middle), while the others withdraw (right). [Courtesy of Damisah et al., Science Advances.]
Joint first authors Eyiyemisi Damisah and Robert Hill used 2Phatal in transgenic mice with fluorescently labeled microglia and astrocytes. The authors targeted a given neuron with 2Phatal, then watched with time-lapse two-photon microscopy. Within two to three hours, several microglia and astrocytes extended processes toward the dying cell. Initially, these processes intermingled, but a single microglia quickly “won out,” migrating toward the dying cell and swallowing it (see image above). Meanwhile, the other phagocytes drew back. The victorious microglia also covered and digested nearby dendrites (see video below).
Over and Done. Time-lapse of a microglia (green) moving in and engulfing a dying neuron (yellow). In reality, this took 24 hours. [Courtesy of Damisah et al., Science Advances.]
The dendritic arbor was another matter. As this fine neuropil degenerated into small apoptotic bodies, delicate astrocytic processes crept out to encapsulate and digest them (see image below). Unlike microglia, the astrocyte cell bodies did not move.
Because dendritic arbors can be quite large, extending long distances from the cell body, and are themselves surrounded by astrocytes, it makes sense that these cells would be tasked with tidying them up, Grutzendler noted. He believes the findings belie the idea that astrocytes are “nonprofessional phagocytes.” “They’re not just a backup phagocyte. We think they’re actively involved in the phagocytic process every time. They have a specialized function,” he told Alzforum.
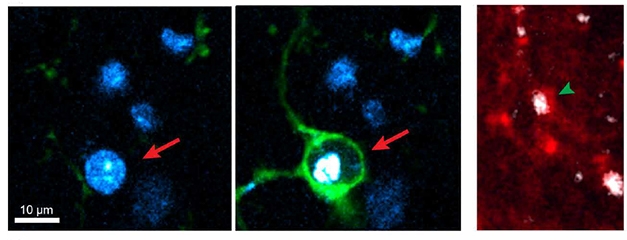
Specialized Cleanup. Microglia (green) engulf the soma of a dying neuron (arrow, left), while astrocyte processes (red) swallow degenerating neurites (arrowhead, right). Dying cell is white, nuclei blue. [Courtesy of Damisah et al., Science Advances.]
Is this coordinated cleanup response specific to 2Phatal? The authors examined two other types of cell death, developmental and induced by viruses. In postmortem sections from developing mouse brain, microglia likewise surrounded apoptotic cell bodies and nearby processes, while astrocytes mopped up distant debris, with a sharp boundary between the two (see image at top of story). The same thing occurred in the cortices of mice infected by a virus.
Next, the authors searched for the mechanism behind this phagocytic response. Two tyrosine kinase receptors, Axl and Mertk, reside in the cell membrane of both astrocytes and microglia. These receptors mediate phagocytosis by sensing signals on the surface of infected or dying cells (Chung et al., 2013; Fourgeaud et al., 2016; Tufail et al., 2017). To probe their role, the authors induced 2Phatal in mice lacking them. In Axl knockouts, they saw no change in clearance efficiency. In Mertk knockouts, however, the time it took to clear the doomed neuron doubled from 48 to 96 hours. The lag occurred because microglia took longer to respond to dying cells; once they had contacted a shrunken neuron, the phagocytes devoured it at normal speed.

Power in Numbers. In the absence of microglia, astrocytes (green) work together to form a barrier (left) around a dying neuron (red, arrow), and gradually disintegrate it (right). [Courtesy of Damisah et al., Science Advances.]
Does Mertk also control the interplay between microglia and astrocytes? The two cell types appear to communicate, because the faster microglia target a dying neuron, the less likely astrocytes are to extend their own processes toward it. Indeed, when the authors knocked Mertk out only in microglia, inhibiting their response, astrocytes attempted to compensate. Astrocytes recruited lysosomes into their processes and extended them to touch the dying neuron and digest it piecemeal. Similarly, when the authors completely ablated microglia from mouse brain with a toxin, the astrocytes around a dying neuron worked together to remove it. Their processes intermingled to wall off the neuronal corpse and nibble away at it (see image above). In both cases, the process was slower than microglial cleanup, taking twice as long.
“In the absence of microglia, astrocytes can take up the burden of cell body removal,” Damisah noted. Nonetheless, astrocyte cleanup appears to be less efficient than microglial. Like microglia, astrocytes also depend on Mertk to recognize dying cells, since they fail to respond when they lack this receptor. Intriguingly, Mertk mutations have been implicated in neurodegeneration (Gal et al., 2000).
Finally, the authors studied what happens with aging. In 26- to 28-month-old mice subjected to 2Phatal, removal of dead cells was slow, increasing from a maximum of about 32 hours in young mice to 64 hours. However, because the authors could not age reporter mice that long, they were unable to perform live imaging of glia to determine which part of the process was delayed: recognition, phagocytosis, or coordination between phagocytes.
Delayed removal of dead neurons in aging brains could potentially harm surrounding tissue, perhaps triggering an inflammatory process, Damisah speculated. “If there’s too much debris in the brain, does the whole system get clogged up?” she asked. In future work, she will investigate how these lingering corpses affect nearby cells and synapses. In fly brains, the failure to remove apoptotic neurons triggers neurodegeneration with age (Etchegaray et al., 2016).
Senescent cells are known to build up in aged brain, and likewise have been linked to neurodegenerative disease (Sep 2018 news). James Kirkland at the Mayo Clinic in Rochester, Minnesota, studies senescence. He believes the slowdown in phagocytic clearance with age could be relevant to that. “Potentially, all of these changes could be linked in some way to the accumulation of senescent microglia and astrocytes,” he wrote to Alzforum.—Madolyn Bowman Rogers
References
News Citations
Paper Citations
- Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, Frenkel D, El Khoury J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016 May 11;36(19):5185-92. PubMed.
- Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, Nabekura J, Sato K, Okajima F, Takebayashi H, Okano H, Koizumi S. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun. 2017 Jun 22;8(1):28. PubMed.
- Hill RA, Damisah EC, Chen F, Kwan AC, Grutzendler J. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat Commun. 2017 Jun 16;8:15837. PubMed.
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013 Dec 19;504(7480):394-400. Epub 2013 Nov 24 PubMed.
- Fourgeaud L, Través PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagórska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016 Apr 14;532(7598):240-244. Epub 2016 Apr 6 PubMed.
- Tufail Y, Cook D, Fourgeaud L, Powers CJ, Merten K, Clark CL, Hoffman E, Ngo A, Sekiguchi KJ, O'Shea CC, Lemke G, Nimmerjahn A. Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia. Neuron. 2017 Feb 8;93(3):574-586.e8. Epub 2017 Jan 19 PubMed.
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000 Nov;26(3):270-1. PubMed.
- Etchegaray JI, Elguero EJ, Tran JA, Sinatra V, Feany MB, McCall K. Defective Phagocytic Corpse Processing Results in Neurodegeneration and Can Be Rescued by TORC1 Activation. J Neurosci. 2016 Mar 16;36(11):3170-83. PubMed.
Further Reading
News
- CD22 Suppresses Microglial Phagocytosis—A New Therapeutic Target?
- CD22 Suppresses Microglial Phagocytosis—A New Therapeutic Target?
- Could CD33 Be the Microglial Target for Stimulating Phagocytosis?
- Deleting CD33 Benefits Mice—If Their Microglia Express TREM2
- Not Just “Glia”: Astrocytes Are Specialized Eating Machines, Not Oligodendrocyte Siblings
- To Monitor Neurons, Microglia Talk With the Boss, aka the Soma
Primary Papers
- Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, Grutzendler J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv. 2020 Jun;6(26):eaba3239. Epub 2020 Jun 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
Washington University in St. Louis
The group of Jaime Grutzendler has recently developed a cutting-edge technique, 2Phatal, allowing researchers to induce apoptosis of individual neuronal cells using a photochemical reaction. From the same group, Damisah and colleagues now took advantage of this method to study, in real time, the phagocytosis of dying neurons mediated by microglia and astrocytes. It is known that both microglia and astrocytes are phagocytic cells (Chung et al., 2013) and express receptors for apoptotic signals, such as extracellular phosphatidylserine. However, whether their phagocytic functions differ in terms of activation dynamics, efficiency, or spatial distribution is unknown.
This study first demonstrates that microglia become readily activated upon apoptosis induction and quickly migrate toward the dying neuron. Microglia exhibit a marked preference for the primary dendrites and the neuronal soma, which is fully engulfed within six hours. Conversely, astrocytes display a relatively slower activation pattern, and seem to be primarily engaged in the phagocytosis of distal dendrites. These complementary effector functions might stem from intrinsic properties of microglia and astrocytes. The first are highly motile cells and rapidly respond to chemoattractant molecules (such as ATP), whereas the latter are relatively immobile and much bigger in size, yet they can occupy larger territories.
The authors have also explored the contribution of two phagocytic receptors, Mertk and Axl, in the phagocytosis of dying neurons. Deletion of Mertk almost doubles the time between the initial microglia engagement and complete removal of the apoptotic corpses. By contrast, deletion of Axl has apparently no effect. This may suggest that Mertk plays important roles in the phagocytosis of dying neurons, whereas Axl is redundant.
Next, the authors determined the contribution of each cell type to the process of neuronal phagocytosis. To do so, they examined mice with complete Mertk deficiency (Mertk knockout), and mice with microglia-specific Mertk deletion (CSF1R-Cre x Mertk flox/flox). Interestingly, microglia-specific Mertk knockout mice do not show improved removal of apoptotic cells compared to the constitutive knockout condition. These data suggest that microglia, rather than astrocytes, are mainly responsible for the phagocytosis of dying cells. Nonetheless, microglia-specific Mertk knockout mice exhibit increased lysosome formation in astrocytes surrounding the apoptotic neurons.
A similar scenario can be observed in mice that underwent microglia depletion via administration of the CSF1R inhibitor, a drug that induces microglia apoptosis. Microglia-depleted mice exhibit a significant delay in the removal of apoptotic cells. Contingently, an increased recruitment of astrocytic filopodia toward the target neurons, eventually forming an envelope around the apoptotic cell body, can be observed. Altogether, these findings indicate that astrocytes are trying to compensate for the loss of microglial phagocytosis; however, microglia are required for efficient scavenging of apoptotic corpses within the brain parenchyma.
Lastly, the authors demonstrated that removal of dead cells is significantly delayed in aged mice compared to young-adult mice. This indicates that aged microglia display an exhausted phagocytic activity, possibly owing to their relatively long life span and progressive accumulation of lipids and ROS exposure. Indeed, it is currently believed that the age-dependent loss of microglia functions may facilitate the onset of neurodegenerative disorders, like Alzheimer’s disease.
To sum up, this elegant study reveals a coordinated and finely orchestrated action of both microglia and astrocytes during cell death. These two cell types seem to play complementary, non-redundant functions, and occupy distinct territories when a neuronal cell undergoes apoptosis.
Microglia rapidly migrate toward the neuronal soma, which is engulfed in a few hours. At the same time, neighboring astrocytes exert their phagocytic action at more distance, where they help remove distal dendrites and small debris. Furthermore, astrocytes appear to form a sort of barrier circumventing the injured microenvironment, thus limiting the diffusion of intracellular molecules that could induce tissue inflammation, such as DNA and ROS.
It will be interesting to see how this technique will be exploited to study microglia- versus astrocyte-mediated phagocytosis in pathological settings. One suggestion for the authors is that the predominant role of Mertk over Axl may reflect different expression levels of these receptors in homeostatic microglia, rather than functional differences. Mertk is highly expressed in microglia under homeostasis, whereas expression of Axl is negligible. However, Axl could be upregulated under pathologic conditions. Future studies are needed to address whether Axl is important for microglia-mediated phagocytosis in models of brain diseases.
References:
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013 Dec 19;504(7480):394-400. Epub 2013 Nov 24 PubMed.
University of Cambridge
The sophisticated “2Phatal” technique developed by Jaime Grutzendler's group has allowed them to investigate how dead neurons are cleared in vivo in real time. Damisah, Hill and colleagues show that after inducing apoptosis in specific neurons by photobleaching Hoechst 33342-stained neuronal nuclei, microglia specialize in removing the cell bodies and proximal dendrites whereas astrocytes specialize in removing apoptotic bodies along axonal debris, all within hours. Cleared neurons leave no sign, as they are entirely degraded. Importantly, this specialization is not only observed for natural apoptotic settings, but is also observed after a non-specified mode of death is induced by viral toxicity, where death is neither apoptotic (no active caspase-3) nor necroptotic (no MLKL, although visualizing phospho-MLKL might have provided a more specific indicator).
The key question is whether these mechanisms govern what happens in neurodegenerative diseases, especially those with protein inclusions?
How neurons die in neurodegenerative diseases is a conundrum. Apoptosis is unlikely to be the major cause of death since apoptosis inhibitors do not prevent cell death in several neurodegenerative disease model systems, implying that other mechanisms predominate. One alternative mechanism (dubbed phagoptosis) has been proposed, whereby microglia become activated to recognize live neurons that display “eat me” signals such as phosphatidylserine (PtdSer) due to various insults (Neher et al., 2013; Brown and Neher, 2014), not least of which is filamentous P301S tau (Brelstaff et al., 2018; Aug 2018 News).
We showed that microglia consume live cell bodies of neurons with filamentous P301S tau in vitro, since blocking phagocytosis leaves live neurons, and in the brains of P301S tau mice, tau inclusions are found in microglia that form intimate contacts around such neurons. In this case, neurons may be cleared, but indigestible tau inclusions are left behind. These could alter the way microglia handle the next events.
Are there common signals that draw the microglia to insulted-but-viable neurons and apoptotic neurons? One candidate might be exposed PtdSer. The Grutzendler group showed previously that calcium rises within two hours after irradiation, before pyknosis sets in around six hours (Hill et al., 2017). Cytoplasmic calcium regulates PtdSer exposure via the phospholipid scramblase TMEM16F (Suzuki et al., 2010; Watanabe et al., 2018), which accelerates phospholipid shuttling between the two bilayers; this is balanced by the flippases ATP 11A/C, which shunt the phospholipids to the internal leaflet of the membrane (Nagata et al., 2016). Notably, flippase activity is inhibited by calcium and is also ATP-dependent, so will be further inhibited by lower ATP levels, a possibility suggested by the mitochondrial fragmentation shown by Grutzendler’s group to occur as part of the apoptotic process in the irradiated neurons.
Another mechanism may be that described by Cserép et al. (2020) where microglia form focal contact sites on cell bodies specifically involving the microglial purinergic (ATP/ADP) P2Y12 receptor and neuronal K+ channels of the Kv2.1/2.2 family. Interestingly, these contacts were less focused after an ischemic insult, which the authors suggest could either enforce neuroprotection by microglia or lead to “isolation and phagocytosis of dying neurons in case terminal neuronal injury occurs.”
Two observations make the correlation between the data provided by the Grutzendler group and phagoptosis less clear. First, in aged mice, the whole clearing process is delayed by two to three days although the apoptotic process seems to be intact and the extension of microglial protrusions appears to be unperturbed. The glial receptor Mertk, which mediates the phagocytic process by microglia in the 2Phatal system, also seems to be intact. If the correct signals are still produced on the neuronal surface, then other factors yet to be identified must assume control. Second, when microglia were ablated using PLX3397, astrocytes undertook the phagocytic role of microglia and eventually consumed the cell body, albeit in a piecemeal fashion rather than by engulfment of the entire cell body. We have not observed tau aggregates inside astrocytes in the P301S tau mouse model, but perhaps ablation of microglia might reveal a similar mechanism at play.
It will be fascinating to find out what pathways microglia and astrocytes follow when they encounter neurons that express neurodegenerative disease proteins due to proteostasis. Do they kill the neurons and then remove the debris, or do they engulf them alive and kill them after internalization?
And is the same dichotomy of roles between microglia and astrocytes preserved? If so, why are there abundant tau deposits in astrocytes but deposits of tau are rarely observed in microglia in several human tauopathies? Could tau “ghost” tangles found in Alzheimer’s disease be partly the post-microglial indigestible remains? While the Grutzendler group is in a good position to answer some of these questions, the connection between tau and the mode of cell death remains to be resolved.
References:
Neher JJ, Emmrich JV, Fricker M, Mander PK, Théry C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):E4098-107. Epub 2013 Oct 7 PubMed.
Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014 Apr;15(4):209-16. PubMed.
Brelstaff J, Tolkovsky AM, Ghetti B, Goedert M, Spillantini MG. Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep. 2018 Aug 21;24(8):1939-1948.e4. PubMed.
Hill RA, Damisah EC, Chen F, Kwan AC, Grutzendler J. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat Commun. 2017 Jun 16;8:15837. PubMed.
Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010 Dec 9;468(7325):834-8. Epub 2010 Nov 24 PubMed.
Watanabe R, Sakuragi T, Noji H, Nagata S. Single-molecule analysis of phospholipid scrambling by TMEM16F. Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):3066-3071. Epub 2018 Mar 5 PubMed.
Nagata S, Suzuki J, Segawa K, Fujii T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016 Jun;23(6):952-61. Epub 2016 Feb 19 PubMed.
Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, Orsolits B, Molnár G, Heindl S, Schwarcz AD, Ujvári K, Környei Z, Tóth K, Szabadits E, Sperlágh B, Baranyi M, Csiba L, Hortobágyi T, Maglóczky Z, Martinecz B, Szabó G, Erdélyi F, Szipőcs R, Tamkun MM, Gesierich B, Duering M, Katona I, Liesz A, Tamás G, Dénes Á. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020 Jan 31;367(6477):528-537. Epub 2019 Dec 12 PubMed.
Institute of Experimental Medicine
Our knowledge about the diversity of cellular phenotypes and responses in the brain has increased substantially in recent years due to advances in single-cell analysis and other approaches. In line with this, there is an increasing need to uncover the complexity of intercellular interactions in health and disease, which are difficult to study ex vivo. While earlier studies have predominantly focused on communication between neurons, recent advances in modern imaging tools, genetic models, and photochemical approaches now enable more precise visualization of interactions between different cell types in vivo. Such studies—especially those pursuing glia-glia or glia-neuron interactions—show that even seemingly well-defined processes, like elimination of terminally injured cells from the brain, are far more complex than previously believed. A major challenge here is to observe complex biological processes that take place at different spatial and temporal scales in order to understand the most important steps of the fine-tuned intercellular communication, which cannot be untangled by using single-cell analysis or high-resolution anatomy.
In this very interesting paper, Damisah et al. combined powerful in vivo two-photon imaging with photochemical cell ablation to show that microglia and astrocytes eliminate different parts of apoptotic neurons, which takes place via precisely regulated cell-cell interactions. The cornerstone of these studies is a bright approach the authors developed. It uses femtosecond-pulsed laser to bleach a nucleic acid binding dye within experimentally targeted cells. This allows tracking of glial responses in the vicinity of dying neurons. The authors found that astrocytes and microglia do different jobs, but their responses are tightly linked. While microglia phagocytosed dendrites, cell bodies, and nuclei of apoptotic neurons following directed migration to these cells, astrocytes engulfed diffuse apoptotic bodies derived from the extensive dendritic arbors without dislocation of their cell body.
Collaborative removal of dead cells appears to require the presence of receptor tyrosine kinase Mertk that senses translocated phosphatidylserine on the outer membranes of dying cells. In fact, Mertk deficiency resulted in delayed recognition of apoptotic neurons by microglia, and a failure of astrocyte polarization toward dying cells. The authors also show that astrocytes can partially substitute microglia in phagocytosing larger parts of terminally injured cells. For example, they can isolate cell bodies with their processes in case microglia are not around—although phagocytosis works way less effectively in this case. In the absence of normal microglial response, astrocytes also display marked polarization of lysosomes at the contact points with the dying cells. Finally, the authors show that the efficiency of phagocytic removal of cell nuclei is reduced in aged mice, suggesting that interactions between astrocytes and microglia in phagocytic responses could be important in age-related diseases of the brain.
The well-designed experiments presented in the paper raise a number of questions, many of which extend beyond the scope of these studies.
Microglial phagocytosis is believed to be crucial to maintain brain tissue homeostasis. Studies using in vivo or ex vivo imaging of phagocytosis also show that phagocytic events are relatively fast and frequent, hence, histological studies most likely underestimate the number of phagocytic events. The efficiency of phagocytosis is important to prevent disease: Microglial phagocytosis is known to be chronically impaired in different pathological conditions such as epilepsy and stroke, resulting in accumulation of apoptotic cells and induction of inflammation (Neumann et al., 2009; Sierra et al., 2014; Sierra et al., 2010).
Currently it is not well understood how these processes are regulated and whether other cell types, for example astrocytes, could contribute to phagocytosis or shape microglial responses. A few years ago, results of pharmacological microglia depletion studies were quite surprising in this regard: Microglia can be eliminated from the mouse brain by using CSF1R inhibitors for several weeks without any obvious detrimental consequences (Elmore et al., 2014). However, an absence of microglia turned out to be highly detrimental after experimental stroke, when there is excessive neuronal injury (Szalay et al., 2016). The apparently healthy phenotype of microglia-depleted mice in the absence of experimental injury may suggest that astrocytes could effectively substitute microglia in maintaining tissue homeostasis if there are not too many dying cells around.
Thus, it remains important to determine in future studies to what extent astrocytes are capable of substituting microglia to phagocytose apoptotic cells. The paper shows that in the normal brain, 90 percent of the nuclei of apoptotic neurons were removed within 24 hours by microglia, which fell to 0 percent when microglia were depleted by the CSF1R inhibitor PLX3397. This, together with tightening of the astrocytic envelopment around apoptotic neurons and polarization of lysosomes, collectively suggest that astrocytes, instead of fully engulfing dying cells (i.e. isolating them from their environment) may only be capable of nibbling away parts of neighboring cells through a process called trogocytosis (Gong et al., 2019). They may remove diffuse microdebris, whereas efficient phagocytosis of large particles, like cell bodies or nuclei, requires the presence of functional microglia.
The observation that microglia tended to specialize in engulfing dendritic branches closest to the soma and neuronal cell bodies may be very relevant for our recent findings, namely microglial somatic junctions, through which microglia sense changes in neuronal activity and shape the fate of injured neurons (see Cserép et al., 2020; Dec 2019 news). These hot spots in the neuronal somatic membrane are identified by the vicinity of mitochondria, exocytosis-promoting Kv2.1 clusters, and other nanoscale assemblies. This allows released ATP and other substances to attract microglial processes, providing an ideal starting point for phagocytosis.
Because neuronal injury triggers an increase in microglial process coverage of neuronal somata via purinergic mechanisms at somatic junctions, we have proposed that these sites are ideal for microglia to rapidly identify dying cells (e.g., based on the release of mitochondrial-derived signals or altered ATP release, followed by activation of membrane receptors upon contact formation) and initiate phagocytosis. Similar processes were observed in the vicinity of dying neurons due to neurotropic virus infection, where microglial P2Y12 (a purinergic receptor that recognizes ADP that is rapidly formed upon ATP release) was essential for microglial chemotaxis and effective phagocytosis (Fekete et al., 2018).
In case phagocytosis is initiated, recruited microglial processes may sense flipped phosphatidylserine via Mertk or other receptors, followed by the dislocation of microglial cell bodies. Interestingly, the authors show that in Mertk−/− mice, the timing of initial microglia process engagement with the dying cells, rather than the overall duration of the phagocytic event, was delayed. Thus, it will be very interesting to study astrocyte-microglia interactions in the vicinity of somatic microglial junctions, where processes of astrocytes are also frequently observed. Territorial segregation of glial processes during cell corpse engulfment shown in the paper indicates precise spatial and temporal control of these actions between astrocytes and microglia, although the underlying mechanisms remain to be identified. Supporting this, the authors show that microglia engagement with the soma of neurons was important for determining the degree of the subsequent astrocyte response, specifically, astrocyte lysosome polarization.
It also remains to be defined whether there is a role for astrocytes in the elimination of dying glial cells apart from neurons. Although astrocytes have long been known for their ability to exert phagocytic activity (Morizawa et al., 2017), apoptotic cells in the brain are believed to be eliminated mostly by microglia. However, there are special conditions, when astrocyte-mediated phagocytosis of glial cells may be particularly important and is possible to study. For example, it is not understood how apoptotic microglia are eliminated from the brain after genetic or pharmacological microglia depletion. Astrocytes appear to be ideal candidates to remove apoptotic microglia or other cells. Given that microglia are self-renewing and long-lived, astrocyte-microglia interactions may also provide a tempting opportunity for astrocytes to control microglial population dynamics in the CNS through soluble factors, membrane-membrane interactions, or phagocytosis of dysfunctional microglia.
Another interesting question could be whether astrocytes are able to instruct microglia to change their phagocytic activity or in turn, whether interactions with microglia would trigger astrocyte phagocytosis in compartments, which are not accessible to microglial processes. In this regard, astrocyte-microglia-neuron interactions should be explored in more detail at synapses and somatic microglial junctions among many others. The perivascular niche could also represent an interesting site for such interactions, for example concerning the removal of injured endothelial cells, which are less accessible for microglia. This may be particularly interesting in age-related brain diseases, where neurodegeneration parallels altered reactivity of astrocytes and microglia, in line with changes in vascular responses such as reduction in cerebral perfusion or functional hyperemia.
The sequence of events, including precise timing of astrocyte and microglia responses upon the detection of apoptotic and necrotic cells, will also be important to study. While microglial processes move faster and extend further than those of astrocytes, it is possible that signals released from nearby dying cells may trigger astrocyte response even earlier than in microglia. Timing of such responses could be crucial to limit tissue injury and inflammation. As apoptotic processes differ from forms of cell necrosis in the extent of inflammation induced in the surrounding tissue, impaired phagocytosis of apoptotic or necrotic cells may also trigger excessive inflammation in case microglia are absent or dysfunctional. This may be partially due to the fact that astrocytes respond to the presence of apoptotic or necrotic cells, but cannot effectively remove them.
Prolonged exposure to dead cells will likely increase leakage of damage-associated molecular patterns or alarmins such as HMGB1, DNA, IL-1α, etc. that could trigger excessive inflammation via acting on microglia, astrocytes, or other cells. As such, conditions which are associated with impaired microglial phagocytic activity (either excessive phagocytosis or lack of proper phagocytic response) likely include alterations in microglia-astrocyte interactions. The paper shows that corpse clearance becomes inefficient in advanced aging.
In the aged brain, in response to inflammatory stimuli that take place in the brain or under systemic inflammatory conditions due to infection, injury, or chronic disease, microglial phenotypes are altered. This may make microglia less able to phagocytose, or, may even boost microglial phagocytic activity that could parallel inappropriate target recognition (such as increased synapse elimination in AD or excessive phagocytosis of injured neuronal cell bodies after stroke or brain trauma). Astrocytes are likely to respond to altered microglial activity (Liddelow et al., 2017) under these conditions. On the other hand, stimuli of vascular origin may affect astrocytes earlier than parenchymal microglia and hence, astrocytes may be drivers of altered microglial phagocytic or other responses in the neurovascular unit.
Finally, regulation of extracellular space and interstitial fluid volume and control of extracellular matrix deposition are critically important in the brain to maintain ion balance and to shape the activity of neurons and glial cells, among other processes. In this regard, the observations that NG2 glia appeared to fill the space left following corpse removal in addition to the more obvious reaction of astrocytes and microglia could be interesting to study. It is likely that multicellular interactions are required to handle the space left after removal of apoptotic cells. This may be very relevant for both the regenerative processes after acute brain injury and in chronic neurodegenerative disorders. Taken together, the mechanisms of intercellular interactions revealed in this paper should be extensively studied in different compartments of the brain.
References:
Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009 Feb;132(Pt 2):288-95. PubMed.
Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, Phagocytosis, and Inflammation: How Never-Resting Microglia Influence Adult Hippocampal Neurogenesis. Neural Plast. 2014;2014:610343. Epub 2014 Mar 19 PubMed.
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010 Oct 8;7(4):483-95. PubMed.
Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014 Apr 16;82(2):380-97. PubMed.
Szalay G, Martinecz B, Lénárt N, Környei Z, Orsolits B, Judák L, Császár E, Fekete R, West BL, Katona G, Rózsa B, Dénes Á. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016 May 3;7:11499. PubMed.
Gong J, Gaitanos TN, Luu O, Huang Y, Gaitanos L, Lindner J, Winklbauer R, Klein R. Gulp1 controls Eph/ephrin trogocytosis and is important for cell rearrangements during development. J Cell Biol. 2019 Oct 7;218(10):3455-3471. Epub 2019 Aug 13 PubMed.
Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, Orsolits B, Molnár G, Heindl S, Schwarcz AD, Ujvári K, Környei Z, Tóth K, Szabadits E, Sperlágh B, Baranyi M, Csiba L, Hortobágyi T, Maglóczky Z, Martinecz B, Szabó G, Erdélyi F, Szipőcs R, Tamkun MM, Gesierich B, Duering M, Katona I, Liesz A, Tamás G, Dénes Á. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020 Jan 31;367(6477):528-537. Epub 2019 Dec 12 PubMed.
Fekete R, Cserép C, Lénárt N, Tóth K, Orsolits B, Martinecz B, Méhes E, Szabó B, Németh V, Gönci B, Sperlágh B, Boldogkői Z, Kittel Á, Baranyi M, Ferenczi S, Kovács K, Szalay G, Rózsa B, Webb C, Kovacs GG, Hortobágyi T, West BL, Környei Z, Dénes Á. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. 2018 Sep;136(3):461-482. Epub 2018 Jul 19 PubMed.
Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, Nabekura J, Sato K, Okajima F, Takebayashi H, Okano H, Koizumi S. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun. 2017 Jun 22;8(1):28. PubMed.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 26;541(7638):481-487. Epub 2017 Jan 18 PubMed.
Boston University School of Medicine
The Grutzendler group visualized spatiotemporal resolution of efferocytosis including sensing, engulfing and digesting the neuronal corpse by microglia and astrocytes by photochemically and virally induced single-cell-targeted ablation of neurons in the live animal brain. The study showed that clearance of most (>80 percent) dying neurons is orchestrated by microglia and astrocytes, and Mertk is an important regulator of timing phagocytic engagement. Interestingly, astrocytes take over a microglial role in their absence.
Based on what’s shown in this paper, i.e., that the cell-death process in neurons is not altered by aging, it is possible that glial cells lose their functions over aging, although the mechanism of aging in glia needs to be explored. In the aging brain, if microglia are delayed in recognition or initiation of phagocytosis and/or astrocytes are in a prolonged clearance process due to defective microglial function thus performing a compensatory role of microglia, then this could increase multiple and multistep risks that lead to neurodegeneration.
Due to the defective efferocytosis in glia, several things could happen:
This study supports the hypothesis that stimulating glial phagocytosis could be beneficial to slow down or reverse aging-associated and/or neurodegenerative diseases. For example, in AD it’s been widely studied that TREM2 knockout in mice and risk variants (e.g., R47H) in humans impairs phagocytosis and sensing of lipids that accumulate during Aβ deposition and apoptosis of neurons, which may be associated with decreased attraction to amyloid plaques (Wang et al., 2015; Kleinberger et al., 2014). Overexpression of TREM2 increases neuronal survival in the presence of Aβ, enhances phagocytosis of Aβ, and prevents loss of memory and learning in animals (Kim et al., 2017). Thus, TREM2 became a target for clinical trials of AD using a monoclonal antibody that activates TREM2 (May 2019 News).
However, it may not work quite so simply just by activating one cell type (e.g., TREM2 in microglia). As this study demonstrated, astrocytes have their own territory and specific functions in efferocytosis, and lysosomal digestion is also critical in this process. While stimulating glia for promoting phagocytosis (uptake, digestion, and efflux) could be beneficial, we also need to consider suppressing inflammation as neurodegeneration is a cumulative synchrony of early and late apoptotic events.
References:
Sachet M, Liang YY, Oehler R. The immune response to secondary necrotic cells. Apoptosis. 2017 Oct;22(10):1189-1204. PubMed.
Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010 Mar;17(3):381-97. Epub 2009 Dec 18 PubMed.
Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991-1045. PubMed.
Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008 Apr;8(4):279-89. Epub 2008 Mar 14 PubMed.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002 Jul 11;418(6894):191-5. PubMed.
Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A. Neuroinflammation Induces Neurodegeneration. J Neurol Neurosurg Spine. 2016;1(1) Epub 2016 Nov 18 PubMed.
Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015 Mar 12;160(6):1061-71. Epub 2015 Feb 26 PubMed.
Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleó A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sánchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, van der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M, Haass C. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014 Jul 2;6(243):243ra86. PubMed.
Kim SM, Mun BR, Lee SJ, Joh Y, Lee HY, Ji KY, Choi HR, Lee EH, Kim EM, Jang JH, Song HW, Mook-Jung I, Choi WS, Kang HS. TREM2 promotes Aβ phagocytosis by upregulating C/EBPα-dependent CD36 expression in microglia. Sci Rep. 2017 Sep 11;7(1):11118. PubMed.
Mayo Clinic
This is a nice story. The technology developed is very impressive, as well. Basically, the investigators developed a single-cell, targeting ablation technology using two-photon-mediated photochemically induced apoptosis (2Phatal) to promote apoptosis only of neurons. Then they investigated phagocytic function of microglia and astrocytes using two-photon live imaging. They compared phagocytosis caused by these two cell types between young and aged mice. In young mice, microglia and astrocytes coordinated phagocytosis well to effectively engulf dead neurons. These two cell types have separate roles and worked efficiently together in the young mice. Microglia engulf the somatic bodies of neurons, while astrocytes remove neuron dendrites. Phagocytosis by astrocytes action depends on microglial activation.
In aged mice, the dead neurons were not removed efficiently. The investigators speculated that reasons could be: 1) defective microglial sensing, 2) loss of coordination between microglia and astrocytes, and 3) impaired microglial phagocytosis. I may be biased but, potentially, all of these changes could be linked in some way to accumulation of senescent microglia and astrocytes.
Make a Comment
To make a comment you must login or register.