TAURIEL Phase 2 Data Published
Quick Links
In the June 13 JAMA Neurology, scientists led by Edmond Teng of Genentech, South San Francisco, California, published results of TAURIEL, a Phase 2 trial of semorinemab in people who had early stage AD. Though the N-terminal tau antibody seemed safe, it slowed neither tau accumulation in the brain nor cognitive decline. Alzforum previously reported on the data at AD/PD last year (Mar 2021 conference news).
During the trial, 422 adults aged 50 to 80 received a placebo or one of three semorinemab doses via monthly infusion for 73 weeks. Researchers collected blood, cerebrospinal fluid, tau PET scans, and structural MRI scans. The primary outcomes were safety and change in the Clinical Dementia Rating-Sum of Boxes. Secondary endpoints were changes in a slew of cognitive tests, while exploratory biomarkers included plasma and CSF tau, standardized uptake value ratios for tau PET, and whole-brain volume.
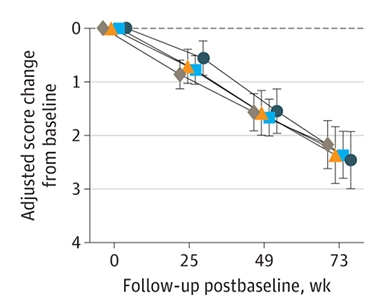
No Change. As judged by the CDR-SB, people taking 1,500mg (orange), 4,500mg (blue), or 8,100 mg (black) of semorinemab declined just as quickly as people taking a placebo (grey). [Courtesy of Teng et al., JAMA Neurology, 2022.]
There was no difference between semorinemab and placebo groups in the rate of decline on the CDR-SB or any of the secondary cognitive tests. Total tau and phospho-tau181 did fall about 10 percent in treated participants, indicating that semorinemab altered tau processing, but this was not dose dependent (see image at left). Oddly, total tau in plasma rose with higher drug doses. The authors believe that this is because the antibodies stabilize tau in the blood.
Despite the modest effect on soluble tau, the antibody did not slow tau accumulation in the brain, as judged by tau PET scans at baseline and weeks 49 and 73 in a subset of 294 participants. Neither did it slow cerebral atrophy as seen by structural MRI.
Semorinemab seemed safe. Teng reported that adverse events were similar in the placebo and treatment groups, with the most common being infusion-related reactions, cold-like symptoms, and falls.
The antibody joins AbbVie’s tilavonemab, Biogen’s gosuranemab, and Eli Lilly’s zagotenemab in falling short in Phase 2 for AD and progressive supranuclear palsy (Nov 2021 news; Jul 2019 news; Dec 2019 news). However, Semorinemab is still being tested in another Phase 2 study of people with moderate AD.
Other tau immunotherapies still in Phase 1 or 2 trials for AD include antibodies against mid-domain tau, such as Roche’s bepranemab, Janssen’s JNJ-63733657, Eisai’s E2814, and Biogen/Neurimmune’s BIIB076, and those targeting hyperphosphorylated tau, including Lundbeck’s Lu AF87908 and Pinteon Therapeutics’ PNT001.—Chelsea Weidman Burke
References
Therapeutics Citations
- Semorinemab
- Tilavonemab
- Gosuranemab
- Zagotenemab
- Bepranemab
- JNJ-63733657
- E2814
- BIIB076
- Lu AF87908
- PNT001
News Citations
- N-Terminal Tau Antibodies Fade, Mid-Domain Ones Push to the Fore
- More Tau Antibodies Bid Adieu; Semorinemab Keeps Foot in Door
- AbbVie’s Tau Antibody Flops in Progressive Supranuclear Palsy
- Gosuranemab, Biogen’s Anti-Tau Immunotherapy, Does Not Fly for PSP
External Citations
Further Reading
Primary Papers
- Teng E, Manser PT, Pickthorn K, Brunstein F, Blendstrup M, Sanabria Bohorquez S, Wildsmith KR, Toth B, Dolton M, Ramakrishnan V, Bobbala A, Sikkes SA, Ward M, Fuji RN, Kerchner GA, Tauriel Investigators. Safety and Efficacy of Semorinemab in Individuals With Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022 Aug 1;79(8):758-767. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California Keck School of Medicine
This negative TAURIEL semorinemab trial in prodromal Alzheimer disease (MMSE 30 to 20) received far more notice than its sibling LAURIET trial (NCT03828747) in mild to moderate AD dementia (MMSE 21 to 16). Yet, in LAURIET there was a highly significant effect on the ADAS-cog11 co-primary after one year of treatment, P value =.0025 (CTAD, Boston, November 2021). There was no significant effect on the ADCS-ADL co-primary or on tau-PET tracer accumulation.
This low P value in an adequate and well-controlled trial is usually considered compelling by statisticians and “persuasive” by the FDA when they are considering a drug for approval. In addition, the ADAS-cog11 drug-placebo mean difference of 2.96 points is larger than in most any other AD trial, and is at a magnitude considered to be a minimum clinically important difference.
Most experts appear unimpressed with LAURIET, however, writing it off as a fluke or random outcome; and there seems to be absent enthusiasm for a confirming or validation trial. Maybe the idea of a treatment intended as a disease modifier showing efficacy in a mild to moderate AD trial runs counter to an orthodoxy that we must treat early stage, prodromal, or preclinical AD with so-called disease-modifying treatments and show biomarker changes in order to appreciate any clinical change.
Make a Comment
To make a comment you must login or register.