Tau, Speckle Wrecker, Disrupts the Nuclear Home
Quick Links
When tau forms aggregates, it yanks other proteins and even RNAs along for the ride. Among these unwitting captives are key components of the cell’s splicing machinery, according to a study published April 7 in Neuron. Researchers led by Roy Parker, University of Colorado Boulder, detected small nuclear RNAs and RNA-binding proteins within tau fibrils in cultured cells and in the mouse brain. These components hail from nuclear speckles—the membraneless organelles that house the spliceosome. Tau fibrils not only disrupted speckle organization, but also snatched pieces from them and made off with the booty into the cytoplasm. These pickings were also found within the cytoplasm in postmortem brain samples from people with tauopathies. The researchers propose that tau’s speckle-wrecking proclivities underlie splicing defects that are reportedly rampant in tauopathies, including Alzheimer’s disease.
- In cells and mice, tau aggregates interact with nuclear speckle components.
- This disrupts mRNA splicing.
- Small RNAs and their binding proteins rerouted to the cytoplasm in cells and in human brain.
“This rigorous study further reinforces that RNA-binding protein aggregation and splicing defects are a core feature of AD and potentially other tauopathies,” commented Nicholas Seyfried of Emory University in Atlanta. He added that the study lends support to previous work showing that several core U1 spliceosome proteins and snRNAs co-aggregate with tau in AD.
Splicing—the ouster of introns and joining of exons—is a critical part of messenger RNA maturation. The splicing machinery, aka the spliceosome, comprises numerous RNA and protein components, held together within dynamic gel-like blobs that are the nuclear speckles. Some of the same spliceosome components have been spotted in aggregates in the AD brain, and in some cases they mingle with tau neurofibrillary tangles (Bai et al., 2013; Hales et al., 2014). Splicing deficits are seen throughout the AD brain as well, suggesting that a toxic relationship between speckle components and tau tangles could derail proper splicing (Oct 2018 news; Hsieh et al., 2019). Such a dangerous liaison would align with prior reports that tau flirts with RNA and RNA-binding proteins, and finds its way into membraneless organelles such as stress granules (Jul 2017 news; May 2016 news).
Against this backdrop, first author Evan Lester and colleagues took a step back and asked: When tau forms aggregates in a cell, does this promote its interaction with RNAs, and if so, which ones? They used a HEK293 biosensor cell line, which expresses truncated versions of tau comprising its four microtubule-binding repeat domains (4R) fused to either a fluorescent donor or acceptor molecule. Upon transfecting the cells with tau fibrils extracted from P301S transgenic mice, the 4R taus are pulled into aggregates, triggering green fluorescence as the donor and acceptor variants are drawn to each other. In their hands, the researchers observed both nuclear and cytoplasmic aggregates upon introduction of tau fibrils. Using fluorescent in situ hybridization, they also spotted RNA abundantly associating with aggregates in both cellular locales. This was also true in neurons in 6-month-old P301S-tau transgenic mice.

Tau Meets Speckle. In HEK cells expressing wild-type tau (top), no tau aggregates (green) form, and the speckle protein SRRM2 (red) stays in the nucleus (blue). In cells expressing P301S tau (bottom), tau aggregates form in the nucleus and cytoplasm (black background). Aggregates co-localize with SRRM2 in both locales (1 and 2, bottom right). [Courtesy of Lester et al., Neuron, 2021.]
Which species of RNA mingled with aggregated tau? By purifying aggregates and sequencing the associated RNA, the researchers found an abundance of noncoding small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs). snRNAs are part of the spliceosome; snoRNAs help modify ribosomal RNAs and transfer RNAs in the nucleolus, which is the birthplace of ribosomes. To a lesser extent, mRNA transcripts also associated with tau, including those encoding a voltage-gated calcium channel complex, histone proteins, centrosome proteins, and proteins involved in splicing regulation. Transfer RNAs and even transposable elements were also detected. The authors found a similar profile of RNA species tussling with tau fibrils in the brains of P301S mice.
Using immunofluorescence, the scientists spotted tau aggregates embedded within speckles in the nucleus, as gauged by co-localization with SRRM2, an RNA-binding protein that resides in these organelles. Strikingly, they also found SRRM2, as well as several other speckle proteins, hooked up with tau aggregates in the cytoplasm. The scientists pegged the C-terminus of SRRM2, which contains an intrinsically disordered domain, as the tau interaction motif.
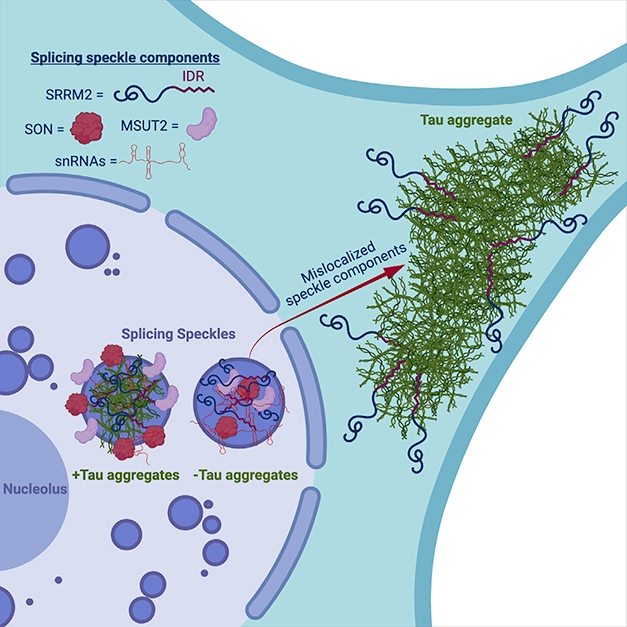
Speckle Wrecker. In this model, tangles (green) co-mingle with snRNAs and proteins that normally belong in nuclear speckles, including SRRM2 (black squiggles), disrupting their form and function. Tau aggregates pull speckle components into the cytoplasm. [Courtesy of Lester et al., Neuron, 2021.]
Did tau’s dalliances with speckles interfere with the function of these membraneless organelles? In a nutshell, yes. Tau messed with all aspects of speckle life. Tau fibrils disrupted physical interactions between proteins within speckles. They altered the spatial organization of speckle components. And they rendered the typically dynamic speckles sluggish and immobile within the nucleus. These disruptions came with splicing defects in the biosensor cells. Among 226 genes known to undergo differential splicing, the researchers detected 305 splicing variations—most commonly intron retentions—upon tau aggregation.

Trapped in Tangles. In a brain sample from a control, SRRM2 (red) resided within speckles in the nucleus (top row). In the brain of a person with corticobasal disease, SRRM2 co-localized with tau tangles (green) in the cytoplasm (bottom row). [Courtesy of Lester et al., Neuron, 2021.]
Biosensor cells are nifty, but does any of this happen in real life? In the brains of P301S-tau mice, but not wild-type, the researchers spied SRRM2 rerouting from the nucleus to the cytosol. The speckle protein was also found mingling with tangles in the cytoplasm in postmortem samples of the left angular gyrus from three people who had had corticobasal degeneration, a primary tauopathy. In another set of samples, SRRM2 remained firmly rooted in the nucleus in four healthy controls, but had strayed into the cytoplasm in four people who had had frontotemporal lobar degeneration and in four who’d had Alzheimer’s disease.
The findings indicate that tau fibrils work their way into nuclear speckles, disrupting their function in the nucleus and even sequestering some of their components in the cytoplasm. Parker’s group is investigating the molecular basis for these shenanigans. For example, does tau join up with speckles by interacting with snRNAs, RNA-binding proteins such as SRRM2, or a combination of both?
Joshua Shulman of Baylor College of Medicine in Houston found the work highly interesting. “The nucleus is emerging as a major cellular target for tau toxicity, with prior work also establishing that tau dysregulates the nuclear lamin and chromatin domains,” he wrote to Alzforum (Frost et al., 2016; Frost et al., 2014).
Benjamin Wolozin and Lulu Jiang of Boston University agreed. “The study adds to a growing body of evidence that tau influences nuclear function,” they wrote to Alzforum. They added that the field needs to uncover how tau aggregates mosey from the cytosol into the nucleus, and how the snRNA, snoRNA, or speckle proteins wind up in the cytosol. Nuclear transport deficits are a predominant feature of tau-related neurodegenerative diseases, and these deficits have been linked to perturbation in the nuclear membrane. “Whether this mis-localization of tau in the nucleus links to nuclear membrane disruption remains an open question,” Jiang and Wolozin wrote.
The study also raises fundamental questions about biology. Do speckle components shuttle in and out of the nucleus under physiological conditions in the human brain, or do they only wash up in the cytoplasm by way of tau? Parker noted that even in the absence of tau fibrils, minute amounts of speckle components trickled into the cytoplasm in HEK cells. Another intriguing question that arises from the study is whether tau might have an intrinsic function as an RNA-binding protein, he said.
Parker is also investigating if tau’s interactions with nuclear speckles influences its fibrillization. “Is there something about the speckle environment that promotes tau fibrillization, or do speckles only recruit aggregates that are already formed?” he wondered.
“It will be important to understand the timing, mechanisms, and importance of tau-mediated nuclear changes relative to other cellular targets in tauopathies,” Shulman added. “These insights may guide ‘nuclear medicine’ for AD in the near future,” he wrote.—Jessica Shugart
References
News Citations
- Mixed Messages: mRNA Splicing Errors May Promote Alzheimer’s
- Tau Hooks Up with RNA to Form Droplets
- Stress Granule Protein Entwines and Misfolds Tau
Paper Citations
- Bai B, Hales CM, Chen PC, Gozal Y, Dammer EB, Fritz JJ, Wang X, Xia Q, Duong DM, Street C, Cantero G, Cheng D, Jones DR, Wu Z, Li Y, Diner I, Heilman CJ, Rees HD, Wu H, Lin L, Szulwach KE, Gearing M, Mufson EJ, Bennett DA, Montine TJ, Seyfried NT, Wingo TS, Sun YE, Jin P, Hanfelt J, Willcock DM, Levey A, Lah JJ, Peng J. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16562-7. PubMed.
- Hales CM, Dammer EB, Diner I, Yi H, Seyfried NT, Gearing M, Glass JD, Montine TJ, Levey AI, Lah JJ. Aggregates of small nuclear ribonucleic acids (snRNAs) in Alzheimer's disease. Brain Pathol. 2014 Jul;24(4):344-51. Epub 2014 Apr 14 PubMed.
- Hsieh YC, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, Dammer EB, Lah JJ, Levey AI, Bennett DA, De Jager PL, Seyfried NT, Liu Z, Shulman JM. Tau-Mediated Disruption of the Spliceosome Triggers Cryptic RNA Splicing and Neurodegeneration in Alzheimer's Disease. Cell Rep. 2019 Oct 8;29(2):301-316.e10. PubMed.
- Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol. 2016 Jan 11;26(1):129-36. Epub 2015 Dec 24 PubMed.
- Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014 Mar;17(3):357-66. Epub 2014 Jan 26 PubMed.
Further Reading
Papers
- Hales CM, Seyfried NT, Dammer EB, Duong D, Yi H, Gearing M, Troncoso JC, Mufson EJ, Thambisetty M, Levey AI, Lah JJ. U1 small nuclear ribonucleoproteins (snRNPs) aggregate in Alzheimer's disease due to autosomal dominant genetic mutations and trisomy 21. Mol Neurodegener. 2014 Apr 28;9:15. PubMed.
Primary Papers
- Lester E, Ooi FK, Bakkar N, Ayers J, Woerman AL, Wheeler J, Bowser R, Carlson GA, Prusiner SB, Parker R. Tau aggregates are RNA-protein assemblies that mislocalize multiple nuclear speckle components. Neuron. 2021 May 19;109(10):1675-1691.e9. Epub 2021 Apr 12 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Boston University School of Medicine
University of Virginia
The presence of tau has been noted in the nucleus for years, although the focus on cytoplasmic tau has biased the field away from the idea that tau might have a nuclear function (Maina et al., 2018; Mansuroglu et al., 2016). Under basal conditions, non-phosphorylated 2N4R tau localizes to the nucleolus, where it presumably contributes to ribosomal genesis and maturation (Maina et al., 2018). Upon stress, nuclear tau gets phosphorylated and mislocalizes out of the nucleolus and spreads throughout the nucleus. The actions of the dispersed nuclear tau have been largely an enigma beyond the general idea that it associates with chromatin, although many different targets of tau action in the nucleus have been identified (Frost et al., 2016; Wheeler et al., 2019; Younas et al., 2020; Cornelison et al., 2019; Eftekharzadeh et al., 2018; Guo et al., 2018).
This current work by Lester et al., emanating from Roy Parker’s laboratory, presents a new take on the actions of “extra-nucleolar” nuclear tau. This work identifies tau alteration of nuclear speckles as a feature of tau aggregation that may contribute to the pathology of tau aggregates. Lester et al. use a tau-seeding model, beginning with HEK 293 reporter cells. The group then identified transcripts that were possibly binding to tau, and discovered many small nuclear and small nucleolar RNAs (snRNAs and snoRNAs). Literature suggests that snRNA associates with a number of nuclear RNA-binding proteins, including MSUT2, SRRM2 and SFPQ, all of which have been shown previously to exhibit abnormal nuclear distribution in Alzheimer brain (Wheeler et al., 2019; Younas et al., 2020; Ke et al., 2012; Tanaka et al., 2018). These proteins are associated with nuclear speckles, which are phase separated droplets.
The Parker group then explored whether the mislocalized tau alters the dynamic nature of the nuclear speckles using FRAP, showing that speckles containing tau were much more static. This suggests an alteration of function. Finally, since studying HEK 293 cells presents a dubious starting point for tau biology, the group looked in a Tg2541 mouse model and in a couple of cases of Alzheimer’s disease or FTLD brain. The group observed redistribution of SRRM2 in the brains of these cases.
The study makes important contributions by confirming the existence of RNA in the complex of tau aggregates, and extends the work with discovery of the co-localization and enrichment of tau aggregates with snRNA and snoRNA in the nucleus and nucleoli speckles. These two important and solid findings open up new windows for further exploration of the function and dynamics of tau in nucleus. These findings build on increasing evidence that tau pathology can impact on nuclear function.
This interesting manuscript continues emerging work demonstrating intimate involvement of tau with RNA and DNA metabolism, and also how tau modulates phase-separation biology (Zhang et al., 2017; Wegmann et al., 2018; Ash et al., 2021; Wolozin and Ivanov, 2019). The manuscript suffers, though, because it begins with HEK 293 cells rather than neurons, particularly central nervous system neurons. The behavior of tau in peripheral cells differs greatly from that in neurons. Starting with HEK 293 cells likely means that the group missed an extensive amount of biology, and might even have gotten some of the dynamic biology wrong . Despite these caveats, this article adds strong evidence to steadily growing body of work showing that tau impacts nuclear function.
The following questions are important to investigate in the future studies:
1. The field needs to uncover how aggregates of tau transport from the cytosol into the nucleus and how the snRNA and snoRNA or nucleoli speckle proteins such as SRRM2 mislocalize into cytosol. Recently, numerous studies have reported that nuclear transport deficits are a predominant feature in tau-related neurodegenerative diseases (Eftekharzadeh et al., 2018; Sheffield et al., 2006). Nuclear envelop disruption and nuclear pore complex dysfunction occurs in parallel with tau aggregation (Frost et al., 2016; Diez and Wegmann, 2020). Whether this mis-localization of tau in the nucleus links to nuclear membrane disruption remains an open question.
2. The manuscript leaves unanswered the question of whether the snRNA/snoRNA recruitment in tau aggregates has functional consequences on gene expression. A prior study by Beth Frost and Mel Feany found that tau promotes neurodegeneration through global chromatin relaxation (Frost et al., 2014). Whether the binding of tau with snRNA/snoRNA contributes to the chromatin abnormalities and related neuronal toxicity needs further demonstration.
3. Finally, the authors have demonstrated the co-localization of snRNA/snoRNA and SRRM2 with p-tau in different tauopathy-related disease including AD, FTLD, and CBD. This sequestration of RNAs and RNA-binding proteins into pathologic aggregates may represent a shared pathophysiological feature across multiple degenerative diseases affecting diverse tissue types, with a common feature being depletion of critical RNA processing factors from the nucleus, leading to changes in RNA processing and gene expression. Such a finding raises the possibility that the affected RNA populations are distinct in different tauopathies, which could be relevant to the differential diagnosis of among tauopathies.
References:
Maina MB, Bailey LJ, Wagih S, Biasetti L, Pollack SJ, Quinn JP, Thorpe JR, Doherty AJ, Serpell LC. The involvement of tau in nucleolar transcription and the stress response. Acta Neuropathol Commun. 2018 Jul 31;6(1):70. PubMed.
Mansuroglu Z, Benhelli-Mokrani H, Marcato V, Sultan A, Violet M, Chauderlier A, Delattre L, Loyens A, Talahari S, Bégard S, Nesslany F, Colin M, Souès S, Lefebvre B, Buée L, Galas MC, Bonnefoy E. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci Rep. 2016 Sep 8;6:33047. PubMed.
Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol. 2016 Jan 11;26(1):129-36. Epub 2015 Dec 24 PubMed.
Wheeler JM, McMillan P, Strovas TJ, Liachko NF, Amlie-Wolf A, Kow RL, Klein RL, Szot P, Robinson L, Guthrie C, Saxton A, Kanaan NM, Raskind M, Peskind E, Trojanowski JQ, Lee VM, Wang LS, Keene CD, Bird T, Schellenberg GD, Kraemer B. Activity of the poly(A) binding protein MSUT2 determines susceptibility to pathological tau in the mammalian brain. Sci Transl Med. 2019 Dec 18;11(523) PubMed.
Younas N, Zafar S, Shafiq M, Noor A, Siegert A, Arora AS, Galkin A, Zafar A, Schmitz M, Stadelmann C, Andreoletti O, Ferrer I, Zerr I. SFPQ and Tau: critical factors contributing to rapid progression of Alzheimer's disease. Acta Neuropathol. 2020 Sep;140(3):317-339. Epub 2020 Jun 23 PubMed.
Cornelison GL, Levy SA, Jenson T, Frost B. Tau-induced nuclear envelope invagination causes a toxic accumulation of mRNA in Drosophila. Aging Cell. 2019 Feb;18(1):e12847. Epub 2018 Nov 9 PubMed.
Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y, Cook C, Miller SJ, Dujardin S, Amaral AS, Grima JC, Bennett RE, Tepper K, DeTure M, Vanderburg CR, Corjuc BT, DeVos SL, Gonzalez JA, Chew J, Vidensky S, Gage FH, Mertens J, Troncoso J, Mandelkow E, Salvatella X, Lim RY, Petrucelli L, Wegmann S, Rothstein JD, Hyman BT. Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer's Disease. Neuron. 2018 Sep 5;99(5):925-940.e7. PubMed.
Guo C, Jeong HH, Hsieh YC, Klein HU, Bennett DA, De Jager PL, Liu Z, Shulman JM. Tau Activates Transposable Elements in Alzheimer's Disease. Cell Rep. 2018 Jun 5;23(10):2874-2880. PubMed.
Ke Y, Dramiga J, Schütz U, Kril JJ, Ittner LM, Schröder H, Götz J. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer's and Pick's disease. PLoS One. 2012;7(4):e35678. PubMed.
Tanaka H, Kondo K, Chen X, Homma H, Tagawa K, Kerever A, Aoki S, Saito T, Saido T, Muramatsu SI, Fujita K, Okazawa H. The intellectual disability gene PQBP1 rescues Alzheimer's disease pathology. Mol Psychiatry. 2018 Oct;23(10):2090-2110. Epub 2018 Oct 3 PubMed.
Zhang X, Lin Y, Eschmann NA, Zhou H, Rauch JN, Hernandez I, Guzman E, Kosik KS, Han S. RNA stores tau reversibly in complex coacervates. PLoS Biol. 2017 Jul;15(7):e2002183. Epub 2017 Jul 6 PubMed.
Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, Vanderburg C, Roe AD, Fan Z, Molliex AM, Hernandez-Vega A, Muller D, Hyman AA, Mandelkow E, Taylor JP, Hyman BT. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018 Apr 3;37(7) Epub 2018 Feb 22 PubMed.
Ash PE, Lei S, Shattuck J, Boudeau S, Carlomagno Y, Medalla M, Mashimo BL, Socorro G, Al-Mohanna LF, Jiang L, Öztürk MM, Knobel M, Ivanov P, Petrucelli L, Wegmann S, Kanaan NM, Wolozin B. TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proc Natl Acad Sci U S A. 2021 Mar 2;118(9) PubMed.
Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. 2019 Nov;20(11):649-666. Epub 2019 Oct 3 PubMed.
Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol. 2006 Jan;65(1):45-54. PubMed.
Diez L, Wegmann S. Nuclear Transport Deficits in Tau-Related Neurodegenerative Diseases. Front Neurol. 2020;11:1056. Epub 2020 Sep 25 PubMed.
Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014 Mar;17(3):357-66. Epub 2014 Jan 26 PubMed.
Barshop Institute for Longevity and Aging Studies/University of Texas Health Science Center
This lovely study from the Parker lab further implicates tau as a splicing disruptor and identifies RNAs that are sequestered within tau aggregates (Raj et al., 2018; Apicco et al., 2019; Hsieh et al., 2019).
While the mechanistic aspect of the paper focuses on splicing, the discovery that mRNAs associated with calcium signaling, histones, and transposable elements are enriched in tau aggregates is quite intriguing. Studies ranging from flies to humans, report that calcium signaling, genomic packaging, and transposable elements are dysregulated in tauopathy and that such dysregulation causally mediates neuronal death. Combined with studies from Songi Han’s laboratory, the data presented here by Lester and colleagues opens new doors for future investigation into the role of physiological and pathological tau on RNA metabolism and consequent changes to cellular function and neurotoxicity (Zhang et al., 2017).
References:
Raj T, Li YI, Wong G, Humphrey J, Wang M, Ramdhani S, Wang YC, Ng B, Gupta I, Haroutunian V, Schadt EE, Young-Pearse T, Mostafavi S, Zhang B, Sklar P, Bennett DA, De Jager PL. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer's disease susceptibility. Nat Genet. 2018 Nov;50(11):1584-1592. Epub 2018 Oct 8 PubMed.
Apicco DJ, Zhang C, Maziuk B, Jiang L, Ballance HI, Boudeau S, Ung C, Li H, Wolozin B. Dysregulation of RNA Splicing in Tauopathies. Cell Rep. 2019 Dec 24;29(13):4377-4388.e4. PubMed.
Hsieh YC, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, Dammer EB, Lah JJ, Levey AI, Bennett DA, De Jager PL, Seyfried NT, Liu Z, Shulman JM. Tau-Mediated Disruption of the Spliceosome Triggers Cryptic RNA Splicing and Neurodegeneration in Alzheimer's Disease. Cell Rep. 2019 Oct 8;29(2):301-316.e10. PubMed.
Zhang X, Lin Y, Eschmann NA, Zhou H, Rauch JN, Hernandez I, Guzman E, Kosik KS, Han S. RNA stores tau reversibly in complex coacervates. PLoS Biol. 2017 Jul;15(7):e2002183. Epub 2017 Jul 6 PubMed.
Baylor College of Medicine / Texas Children's Hospital
This interesting work adds to a growing number of reports that tau can co-aggregate with RNA and RNA-binding proteins. Lester and colleagues further show that this leads to disruptions in nuclear compartments, such as speckles, that have critical roles in mRNA splicing, and therefore, cellular maintenance.
The nucleus is emerging as a major cellular target for tau toxicity, with prior work also establishing that tau dysregulates the nuclear lamin and chromatin domains (Frost et al., 2014; Frost et al., 2016). Is this multipronged nuclear assault by tau connected, and which derangements contribute to tau-mediated neurodegeneration, versus potential downstream consequences? Fruit-fly models expressing human tau may provide some clues, since genetic manipulation of chromatin regulators or nuclear lamins—as well as core spliceosome factors—similarly modify neurodegeneration as shown by Frost et al. and our group (Hsieh et al., 2019).
It will be important to understand the timing, mechanisms, and importance of tau-mediated nuclear changes relative to other cellular targets in tauopathies—these insights may guide “nuclear medicine” for Alzheimer’s disease in the near future.
References:
Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014 Mar;17(3):357-66. Epub 2014 Jan 26 PubMed.
Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol. 2016 Jan 11;26(1):129-36. Epub 2015 Dec 24 PubMed.
Hsieh YC, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, Dammer EB, Lah JJ, Levey AI, Bennett DA, De Jager PL, Seyfried NT, Liu Z, Shulman JM. Tau-Mediated Disruption of the Spliceosome Triggers Cryptic RNA Splicing and Neurodegeneration in Alzheimer's Disease. Cell Rep. 2019 Oct 8;29(2):301-316.e10. PubMed.
Inserm UMR S1172
Lille Neuroscience & Cognition, UMR-S 1172
Lille
Lille Neuroscience & Cognition
Tau aggregates are a “sponge” for numerous biological molecules, including heparan sulfate, proteins, lipids, and nucleic acids such as RNAs. A fundamental question is whether any of these molecules are primary causes of disease by participating in tau seeding and spreading or if they are secondary causes because their sequestration in tau aggregates results in a gain or a loss of function (Galas et al., 2019).
In frontotemporal lobar degeneration, mis-processing and mis-sorting of RNAs contribute to disease. In rare muscular disorders, such as myotonic dystrophy, a mis-splicing of RNAs targeted by the muscleblind-like protein family reduces the diversity of tau isoforms leading to tau aggregation in adults (Fernandez-Gomez et al., 2019; Goodwin et al., 2015; Caillet-Boudin et al., 2014). RNA metabolism and splicing can, therefore, contribute to neurodegenerative disorders, however, the mechanism remains ill-defined.
Here, Evan Lester and colleagues have shown that tau aggregates interfere with different nuclear factors and functions. Tau aggregates from several cellular and animal models were isolated from the cytosol or from nuclei then accompanying RNAs and proteins were identified using orthogonal omics methods. Aggregates were shown to be enriched in poly-A RNA, snoRNAs (U3, U17, and U8), snRNAs (U2 and U1), and some mRNAs coding for nuclear, splicing, and channel proteins. The presence of U1 snRNP is confirmatory of previous data (Bai et al., 2013; Zhu et al., 2020). Characterization of nuclear tau aggregates is novel and they were shown herein to co-localize with the speckle splicing protein SRRM2 and with other speckle components/splicing factors. Moreover, a pathological epitope of tau (phosphorylated at Thr205 and Ser422) co-stained with SRRM2, speckles, and poly(A) RNAs together, helping to demonstrate that tau aggregates are found in the nucleus, localize with speckles, and contain speckle proteins such as SSRM2 and several RNAs.
Interestingly, SRRM2 was also associated with cytosolic tau aggregates, which deplete SSRM2, along with some of its partners such as PNN, SFPQ, and DYRK1A, from nuclear speckles. Does the presence of SRRM2 speckle-associated protein partners in nuclear and cytosolic tau aggregates modify the properties of nuclear speckles and thus mRNA splicing? The authors addressed this question, showing that in cells with tau aggregates several mis-splicing events, especially the retention of more than 1,200 introns encoded by 641 genes, correlated with altered speckle properties, such as their dynamics and spatial organization. Even if many of the components found in tau aggregates were identified in HEK cells (using a FRET cell assay developed by Marc Diamond group), Lester and collaborators validated their findings in human brains. In fact, the loss of nuclear SRRM2 staining was shown to occur in several neurodegenerative diseases, including corticobasal degeneration, Alzheimer’s disease, and frontotemporal lobar degeneration, suggesting a common mechanism associated with tauopathies.
Defective deoxyribo- and ribostasis should now be considered as a consequence of tau aggregation. Tau may protect nucleic acids from stress, whereas in contrast, pathological tau aggregates lead to the defective stasis of nuclear and cytosolic RNAs, the consequences of which remain to be fully characterized. Lester and collaborators make a major contribution toward this better understanding.
References:
Galas MC, Bonnefoy E, Buee L, Lefebvre B. Emerging Connections Between Tau and Nucleic Acids. Adv Exp Med Biol. 2019;1184:135-143. PubMed.
Fernandez-Gomez F, Tran H, Dhaenens CM, Caillet-Boudin ML, Schraen-Maschke S, Blum D, Sablonnière B, Buée-Scherrer V, Buee L, Sergeant N. Myotonic Dystrophy: an RNA Toxic Gain of Function Tauopathy?. Adv Exp Med Biol. 2019;1184:207-216. PubMed.
Goodwin M, Mohan A, Batra R, Lee KY, Charizanis K, Fernández Gómez FJ, Eddarkaoui S, Sergeant N, Buée L, Kimura T, Clark HB, Dalton J, Takamura K, Weyn-Vanhentenryck SM, Zhang C, Reid T, Ranum LP, Day JW, Swanson MS. MBNL Sequestration by Toxic RNAs and RNA Misprocessing in the Myotonic Dystrophy Brain. Cell Rep. 2015 Aug 18;12(7):1159-68. Epub 2015 Aug 6 PubMed.
Caillet-Boudin ML, Fernandez-Gomez FJ, Tran H, Dhaenens CM, Buee L, Sergeant N. Brain pathology in myotonic dystrophy: when tauopathy meets spliceopathy and RNAopathy. Front Mol Neurosci. 2014 Jan 9;6:57. PubMed.
Bai B, Hales CM, Chen PC, Gozal Y, Dammer EB, Fritz JJ, Wang X, Xia Q, Duong DM, Street C, Cantero G, Cheng D, Jones DR, Wu Z, Li Y, Diner I, Heilman CJ, Rees HD, Wu H, Lin L, Szulwach KE, Gearing M, Mufson EJ, Bennett DA, Montine TJ, Seyfried NT, Wingo TS, Sun YE, Jin P, Hanfelt J, Willcock DM, Levey A, Lah JJ, Peng J. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16562-7. PubMed.
Zhu W, Wei X, Wang Y, Li J, Peng L, Zhang K, Bai B. Effects of U1 Small Nuclear Ribonucleoprotein Inhibition on the Expression of Genes Involved in Alzheimer's Disease. ACS Omega. 2020 Oct 6;5(39):25306-25311. Epub 2020 Sep 23 PubMed.
Make a Comment
To make a comment you must login or register.