Tau Immunotherapy for Aβ?
Quick Links
Could antibodies against tau also reduce Aβ pathology? So suggest scientists led by Khalid Iqbal, New York State Institute for Basic Research in Developmental Disabilities, Staten Island. They report that injecting mice with anti-tau antibodies reduced not only total and hyperphosphorylated tau in the brain, while improving memory, but also nudged down levels of the amyloid precursor protein (APP) and cleared Aβ plaques. Appearing in the January 10 Alzheimer's Research & Therapy online, the findings hint that tau immunotherapies could tackle the two major pathological hallmarks of Alzheimer’s. Some researchers were perplexed how this could happen in transgenic mice, while others noted similar observations.
“It’s a very nice paper,” said Einar Sigurdsson, NYU School of Medicine, who was not involved in the study. “To my knowledge, this is the first time a tau antibody has been reported to diminish Aβ pathology in a tau mouse model,” he said. Sigurdsson has unpublished data suggesting that actively immunizing 3xTg mice against tau diminishes both tau and Aβ pathology.
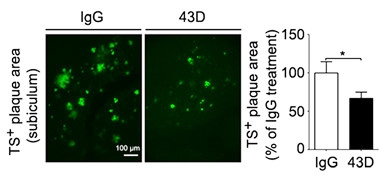
Plaque-Lowering Tau Immunotherapy.
3xTg mice treated with the tau antibody 43D have fewer Thioflavin S+ plaques in the subiculum after six weeks than control transgenics given IgG. [Iqbal et al., 2017. Alzheimers Res Ther.]
In a previous study, Iqbal and colleagues injected 14- to 17-month-old 3xTg mice intraperitoneally with 100 μg of one of two tau antibodies: the 43D antibody that targets amino acids 6-18 at the N-terminus of tau, and 77E9, which recognizes tau 184-195. These mice overexpress Aβ with the Swedish mutation, tau with the P301L mutation, and PSEN1 with the M146V mutation. In the treated animals, the researchers detected a decrease in total and hyperphosphorylated tau over four weeks, as well as an improvement on performance in the Morris water maze. Amyloid plaques and Aβ40 levels trended lower as well (Dai et al., 2014). The researchers wondered if treating younger mice would achieve a more pronounced decrease in Aβ.
To find out, first author Chun-ling Dai and colleagues injected 12-month-old 3xTg mice intravenously with 15 μg of either 43D or 77E9 once a week for six weeks. In this lab, these mice develop plaques around nine months and tangles around 12. Other labs have reported plaques can emerge from six to 12 months in these mice. As controls, Dai injected the transgenic mice with IgG and wild-type mice with either of the tau antibodies or IgG. Twelve to 14 mice were treated per group. Dai assessed spatial learning and memory with the Morris water maze test one day after the sixth injection, and episodic memory with the novel object recognition test a few days after that. Thirty-one days after the sixth injection, animals got a seventh. To see if the effects on memory were long-lasting, Dai tested hippocampus-dependent spatial memory with the novel object location task 100 days after that. The authors also examined tau and Aβ levels in mouse brains using immunohistochemical staining, ELISA, and western blots. Researchers studying these effects were not blinded to the treatment.
As they had reported previously, 3xTg mice treated with either tau antibody improved their performance in the water maze. Animals made a beeline for the target quadrant and swam there longer than transgenic controls. They also spent more time exploring an unfamiliar object than did the IgG-treated control mice. Cognitive benefits lasted at least 100 days after the last immunization. The antibodies also reduced total and hyperphosphorylated tau in the hippocampus relative to control transgenics. Again, these effects were long-lasting. Hyperphosphorylated tau levels stayed low four months after the last injection.
Treatment with the 43D antibody, but not with 77E9, also brought down levels of APP and the Aβ plaque burden. Six days after the last injection, the researchers sacrificed five or six animals in each group to examine their brains immunohistochemically and to test for plaques. APP levels in the forebrain neurons of treated 3xTg mice fell below those in the transgenic controls, with a similar trend in the CA1 region of the hippocampus. What’s more, fewer Thioflavin S-labeled Aβ plaques appeared in the subiculum, the area that carries the largest burden in these mice (see image above).
Interestingly, the subiculum in the 43D mice contained a larger number of activated microglia, which seemed to gather around plaques. This hints that the treatment somehow drew the microglia to digest more of the amyloid. Activation of the complement system might have played a role as well, because 43D tripled C1q and C9 levels. The authors report no microglia or complement effects in the 77E9-immunized animals.
“The N-terminal 43D antibody is incredibly effective at very low doses,” said Michael Agadjanyan, University of California, Irvine, noting that it reduces both total and hyperphosphorylated tau. “I’m amazed.” He said he was surprised an anti-tau antibody could significantly reduce Aβ pathology in aged 3xTg/AD mice. He pointed out that several other papers, notably from Frank LaFerla’s group at UC Irvine, reported that decreasing Aβ42 in 3xTg-AD mice also reduced or delayed the progression of tau pathology (Oddo et al., 2004; Rasool et al., 2013). “These data suggest a crosstalk between Aβ and tau molecules.” He said it would be important to test 43D in mice with only Aβ pathology, to see if the same dose could activate microglia in the absence of the antigen and reduce the plaques.
Iqbal said the evidence hints that 43D reduces plaques by a dual mechanism—less tau leaves neurons healthier so they produce less APP and thus Aβ, while activated microglia clear more plaque. The study points to a reciprocal effect of tau pathology on Aβ, he said. Such a relationship has been studied before. Work from Lennart Mucke’s lab at Gladstone Institute of Neurological Disease, San Francisco, indicates that when tau is reduced in AD mouse models, Aβ no longer causes memory defects, while others have linked intraneuronal tau to toxic effects of Aα on synapses (see May 2007 news; Dec 2011 news). Iqbal thinks interventions that reduce Aβ and tau pathology while improving cognition have a much higher potential for successfully treating Alzheimer’s disease than those that alter either pathology alone. He said his group will humanize the 43D antibody and study its toxicology in preparation for human clinical trials.
Sigurdsson agrees that a dual mechanism may be at play in reducing plaque load. Given that the researchers saw fewer plaques in mice that were immunized just as tau pathology was developing, this is likely a prophylactic effect, rather than one that occurs after significant tau accumulation, he added.
Some scientists cautioned that the numbers of mice per group were low, and the antibody was given at lower doses and for a shorter time than is typical in similar studies. Iqbal said that the antibodies he used are potent. Other scientists commented that since different promoters drive the overexpression of Aβ and tau in this mouse model, the two pathologies are largely independent, making it hard to fathom how treating one can affect the other.
William McEwan, University of Cambridge, U.K., said the microglial effect is plausible, but he wondered about the mechanism that causes APP to fall. He noted that the Thy-1.2 promoter that drives APP expression in this mouse can be regulated by cytokines (Haeryfar and Hoskin, 2004). “I would like to see further confirmation of this result in other systems, or an investigation of how Thy1.2 promoter activity is affected by tau pathology and immunotherapy,” he wrote. Regardless, reductions in tau were seen after a few relatively low and peripherally administered doses of antibody, all of which he found encouraging. “If a mechanistic link between tau immunotherapy and Aβ levels is confirmed it could be highly significant,” he wrote to Alzforum.
Kiran Bhaskar, University of New Mexico, Albuquerque, thought the study excellent. “The elevated levels of phagocytic microglia around the plaque and complement activation is novel [for tau immunotherapy],” he said. He found the long-lasting effects of 43D in suppressing total and hyperphosphorylated tau compelling. Assuming the 43D epitope is conserved in mouse tau, he wondered if the antibody would rescue behavioral impairments or reduce amyloid in a pure amyloid mouse model of AD.—Gwyneth Dickey Zakaib
References
Research Models Citations
News Citations
Paper Citations
- Dai CL, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, Iqbal K. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J Neural Transm. 2014 Sep 19; PubMed.
- Oddo S, Billings L, Kesslak JP, Cribbs DH, Laferla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004 Aug 5;43(3):321-32. PubMed.
- Rasool S, Martinez-Coria H, Wu JW, Laferla F, Glabe CG. Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Aβ deposition and tau pathology in 3xTg-AD mice. J Neurochem. 2013 Aug;126(4):473-82. PubMed.
- Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004 Sep 15;173(6):3581-8. PubMed.
Further Reading
Papers
- Sigurdsson EM. Tau Immunotherapy. Neurodegener Dis. 2016;16(1-2):34-8. Epub 2015 Nov 10 PubMed.
- Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, Cao Y, Snyder B, Zhang B, Nitla M, Hirschfeld G, Barrezueta N, Polson C, Wes P, Rangan VS, Cacace A, Albright CF, Meredith J Jr, Trojanowski JQ, Lee VM, Brunden KR, Ahlijanian M. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One. 2015;10(5):e0125614. Epub 2015 May 1 PubMed.
Primary Papers
- Dai CL, Tung YC, Liu F, Gong CX, Iqbal K. Tau passive immunization inhibits not only tau but also Aβ pathology. Alzheimers Res Ther. 2017 Jan 10;9(1):1. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.