Tangle Density Foretells How Fast a Person’s Brain Will Shrink
Quick Links
A new study supports the idea that deposits of tau predict that a person’s brain will soon wither. Investigators led by Renaud La Joie and Gil Rabinovici, University of California, San Francisco, found that in people with mild Alzheimer’s disease, the intensity of their baseline tau PET dictated their rate of cortical atrophy over the next 15 months. Published in the January 1 Science Translational Medicine, the results suggest tau precedes and drives neurodegeneration. The distribution of tangles in the brain also strongly indicated where the brain would shrink.
- In early AD, the brain shrinks in regions where there are tangles.
- This correlation is stronger in younger patients.
- Tangle-atrophy connection will improve prognosis and trial design.
“This is an elegantly designed study, and a very necessary one,” said Liana Apostolova, Indiana University School of Medicine, Indianapolis.
“To date, many assumptions have been made about the role tau might play to bring on neurodegeneration. However, without longitudinal studies that followed people using neurodegenerative measures downstream of tau, those claims were still hypothetical,” she said.
“That the local distribution of tau pathology can predict the pattern of future cortical atrophy is truly novel and important,” wrote Oskar Hansson, Lund University, Sweden to Alzforum. “It means tau PET could predict clinical outcomes for individual patients, especially if the tau patterns and subsequent atrophy are tied to cognitive symptoms in future studies.”
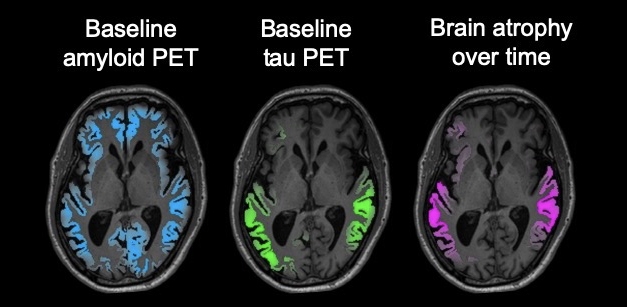
Atrophy Follows Tangles: In a person with early AD, a tau PET scan (green) predicts the location of future brain atrophy (magenta) better than an amyloid scan (blue). [Courtesy of the Rabinovici lab/UCSF.]
In prior cross-sectional studies that have used tau PET, the amount and pattern of tau deposition overlapped more closely with brain atrophy and altered glucose metabolism than did the distribution of Aβ (Iaccarino et al., 2018; Ossenkoppele et al., 2016; Dronse et al., 2017). However, they left unclear which comes first, the tangles or the atrophy?
A recent longitudinal study by Bernard Hanseeuw and colleagues at Massachusetts General Hospital, Charlestown, indicated tau accumulates after Aβ starts to deposit, and tau is the main driver of cognitive decline (Jun 2019 news). But these authors did not examine atrophy. In cross-sectional studies, some of the brain areas that had tau deposits had normal cortical thickness, leading La Joie and colleagues to hypothesize that tangles comes first.
To test this, La Joie, and colleagues analyzed data from 32 participants in an ongoing prospective study at UCSF. Patients ranged in age from 49 to 83; 20 of them were younger than 65. All had a positive amyloid PET scan and signs of early dementia, meeting criteria for mild cognitive impairment or early Alzheimer’s disease (AD). The group was heterogeneous, including six people with a clinical diagnosis of logopenic variant primary progressive aphasia and three with posterior cortical atrophy.
At baseline, each of them underwent a structural magnetic resonance imaging scan followed by PET with both Pittsburgh Compound B (PiB) and 18F-flortaucipir (FTP) to visualize amyloid and tau, respectively. A second MRI scan, about 15 months later, allowed researchers to measure rates of atrophy.
Tau PET predicted atrophy far better than amyloid PET. At the group level, FTP binding appeared highest in the temporo-parietal junction and posterior cingulate/precuneus, as well as dorsal, frontal, occipital, and inferior-medial cortices. Atrophy over this time period strongly associated with baseline global FTP with a correlation coefficient of 0.67, in contrast with correlations of 0.3 or less for atrophy and baseline amyloid PET and baseline cortical thickness.
Further evidence that tau strongly influenced atrophy came from studying regional changes. For individual participants, their baseline tau PET also predicted where their brains would shrink next. Tau explained, on average, 43 percent of the atrophy for each of the 32 patients (see image above). Again, this stood in contrast to the 3 percent correlation between PiB PET and regional atrophy, and even poorer correlation for baseline cortical thickness.
“We expected to see this effect, but we were really surprised at how strong and consistent it was,” La Joie said. The results support the idea that, in the long pathogenic course of early AD, a tau PET signal comes before detectable neurodegeneration, he told Alzforum.
Interestingly, global tau PET and rate of atrophy correlated more tightly in younger than in older people. The authors suggest that this reflects a “purer” tauopathy in patients with earlier onset, in whom AD pathology is likely the main driver of neurodegeneration. In older people, comorbidities such as cerebrovascular disease, other proteinopathies, or aging, could contribute to atrophy, they wrote.
Younger patients had, on average, a higher tau burden and faster atrophy than older patients, reflecting a more aggressive form of disease. The paper does not report the ApoE genotype of these participants, but ApoE4 has long been known to accelerate amyloid AD pathology and lead to earlier onset. A study led by Pedro Rosa-Neto at McGill University, Montreal, and published December 20 in JAMA Neurology, suggests that ApoE4 accelerates medial temporal lobe deposition of tau as well (Therriault et al., 2019).
Does the brain shrinkage predict cognitive decline? A comparison of baseline and follow-up measures of the clinical dementia rating sum of boxes test (CDR-SB) suggested that only atrophy in the precuneus correlated with a worsening cognitive score. This surprised the authors. It’s possible that the relationship in this study is weak because it has only two time points, and used a memory-centric test for a clinically heterogeneous cohort that included people with language- and visuospatial-predominant AD phenotypes. That said, the result jibes with data presented last month at the CTAD conference in San Diego, which saw signs of a time lag between a rising tau-PET signal and cognitive decline (Jan 2020 conference news).
The results could help with clinical trials, La Joie said. Researchers may be able to use tau PET to identify participants who are at the brink of brain shrinkage, stratify them based on the degree of their predicted atrophy, or pinpoint where in the brain to look for atrophy at future time points.
David Holtzman, Washington University in St. Louis, agreed. “This paper nicely shows that tau pathology strongly predicts future focal brain volume loss very well, while the presence of amyloid does not,” he wrote, to Alzforum. “Tau PET imaging should be very useful in clinical trials to enable focusing on specific populations of participants and brain regions, to ask specific questions, and to lower sample size requirements.”
The study was small, and the results, from a research cohort with a large proportion of early onset disease, likely won’t translate to the general population. The authors cautioned that the results may come out differently for presymptomatic or advanced disease stages, where atrophy could be driven by factors other than tau. Future studies will include more follow-up time points, a larger sample with older ages of onset, and additional stages of disease, they wrote.
“This is an excellent paper,” Hanseeuw told Alzforum. “It is important to patients that doctors be able to predict the pattern of deterioration in the future.”—Gwyneth Dickey Zakaib
References
News Citations
- Serial PET Nails It: Preclinical AD Means Amyloid, Tau, then Cognitive Decline
- Tau PET Scans Turn Positive When Amyloid Does; Symptoms Follow
Paper Citations
- Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL, Gorno-Tempini ML, Janabi M, Kramer JH, Lazaris A, Lockhart SN, Miller BL, Miller ZA, O'Neil JP, Ossenkoppele R, Rosen HJ, Schonhaut DR, Jagust WJ, Rabinovici GD. Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer's Disease. Neuroimage Clin. 2018;17:452-464. Epub 2017 Sep 25 PubMed.
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016 May;139(Pt 5):1551-67. Epub 2016 Mar 8 PubMed.
- Dronse J, Fliessbach K, Bischof GN, von Reutern B, Faber J, Hammes J, Kuhnert G, Neumaier B, Onur OA, Kukolja J, van Eimeren T, Jessen F, Fink GR, Klockgether T, Drzezga A. In vivo Patterns of Tau Pathology, Amyloid-β Burden, and Neuronal Dysfunction in Clinical Variants of Alzheimer's Disease. J Alzheimers Dis. 2017;55(2):465-471. PubMed.
- Therriault J, Benedet AL, Pascoal TA, Mathotaarachchi S, Chamoun M, Savard M, Thomas E, Kang MS, Lussier F, Tissot C, Parsons M, Qureshi MN, Vitali P, Massarweh G, Soucy JP, Rej S, Saha-Chaudhuri P, Gauthier S, Rosa-Neto P. Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β. JAMA Neurol. 2020 Apr 1;77(4):470-479. PubMed.
Further Reading
Papers
- Gordon BA, Blazey TM, Christensen J, Dincer A, Flores S, Keefe S, Chen C, Su Y, McDade EM, Wang G, Li Y, Hassenstab J, Aschenbrenner A, Hornbeck R, Jack CR, Ances BM, Berman SB, Brosch JR, Galasko D, Gauthier S, Lah JJ, Masellis M, van Dyck CH, Mintun MA, Klein G, Ristic S, Cairns NJ, Marcus DS, Xiong C, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TL. Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers. Brain. 2019 Apr 1;142(4):1063-1076. PubMed.
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, Ayakta N, Cantwell A, Janabi M, Lauriola M, O'Neil JP, Gorno-Tempini ML, Miller ZA, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain. 2017 Dec 1;140(12):3286-3300. PubMed.
- Xia C, Makaretz SJ, Caso C, McGinnis S, Gomperts SN, Sepulcre J, Gomez-Isla T, Hyman BT, Schultz A, Vasdev N, Johnson KA, Dickerson BC. Association of In Vivo [18F]AV-1451 Tau PET Imaging Results With Cortical Atrophy and Symptoms in Typical and Atypical Alzheimer Disease. JAMA Neurol. 2017 Apr 1;74(4):427-436. PubMed.
- Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, Mellinger TJ, Edwards L, Pham J, Miller BL, Rabinovici GD, Jagust WJ. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019 Feb;85(2):229-240. Epub 2019 Jan 17 PubMed.
Primary Papers
- La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, Chaudhary K, Edwards L, Iaccarino L, Janabi M, Lesman-Segev OH, Miller ZA, Perry DC, O'Neil JP, Pham J, Rojas JC, Rosen HJ, Seeley WW, Tsai RM, Miller BL, Jagust WJ, Rabinovici GD. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020 Jan 1;12(524) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.