Stem Cells Rescue Movement in Monkey Model of Parkinson’s
Quick Links
Researchers are gearing up to start clinical trials that test whether replacement dopamine neurons made from human embryonic stem cells or induced pluripotent stem cells can help patients with Parkinson’s disease (PD). In the longest preclinical trial with the largest number of monkeys yet, scientists led by Jun Takahashi, Kyoto University, Japan, gave their planned protocol a trial run. Dopamine neurons derived from human iPSCs and implanted into the animals’ brains improved the monkeys’ neurological scores and voluntary movements over a year. The findings appeared August 30 in Nature.
- Dopamine neurons from human iPSCs formed no tumors in macaques.
- The grafts integrate into the monkey brain, rescue motor deficits.
- Matching MHC haplotypes between donor and recipient lessens rejection.
“This may be the most convincing study in a Parkinsonian monkey that pluripotent stem cell-derived dopamine neurons can survive over a long period of time and allow behavioral improvement without forming tumors,” said Lorenz Studer, Memorial Sloan Kettering Cancer Center, New York. Because the protocol approximated what Takahashi and others plan to do in humans, it suggests this therapy is almost ready for translation, Studer said.
In the August 30 Nature Communications, the same researchers also reported a way to minimize the immune response to such grafts. They matched part of the donor and host “self” signatures, the major histocompatibility complex expressed on cell surfaces. Microglia and infiltrating leukocytes were likelier to turn a blind eye to matched than unmatched dopamine neurons, and more of them survived implantation. The immunosuppressant tacrolimus—a.k.a. FK506—had the same effect.

Neuronal Grafts: Human-derived dopamine neurons thrive in the brain of a monkey model of PD, testing positive for both dopamine transporter (green) and tyrosine hydroxylase (red). [Courtesy of Takahashi et al., Nature.]
Takahashi plans to start trialing iPSC-derived DA neurons in patients by end of 2018. Dopamine neurons derived from iPSCs or human embryonic stem cells are more readily available than fetal dopaminergic cells, which have seen two decades of trials (Barker et al., 2013). However, iPSC-derived grafts come with safety concerns, not least their tendency to harbor dividing cells that can later form tumors (Trounson 2017; Mar 2017 news). Scientists also debate the value of using a patient’s own cells versus those from a healthy donor, which may be rejected.
In the Nature paper, first author Tetsuhiro Kikuchi and colleagues took fibroblasts or peripheral blood cells from four healthy people and three PD patients and coaxed them to differentiate into midbrain dopamine progenitor cells.
The researchers implanted 28-day-old dopamine progenitor cells bilaterally into the putamen of eight 2-year-old monkeys that had been subjected three months earlier to MPTP, a neurotoxin that wipes out dopaminergic neurons in the substantia nigra. To suppress their immune response, each animal got daily tacrolimus injections from the day before transplantation until the end of the experiment. Three monkeys implanted with vehicle control rather than progenitor cells also received tacrolimus. PET with 11C-PK11195 and (S)-11C-KTP-Me, ligands for activated microglia and inflammation, respectively, registered either no or very mild microglial reaction to the transplants in immunosuppressed animals.
Over the next 12 months, the researchers assessed neurological symptoms—facial expressions, movement in response to stimuli, tremor, posture—and tracked spontaneous movement in 90-minute video recordings taken every quarter. After 12 to 24 months, the researchers sacrificed the animals to see if the cells had integrated into the brain.
Monkeys that received the cell grafts, regardless of whether the cells came from controls or PD patients, improved their neurological scores by 40 to 50 percent. The control group scores rose by 10 percent. After a year, treated monkeys moved three times as much as they had before transplantation; the controls saw almost no improvement. The researchers detected no signs of tumors.
Based on histological examination, the researchers estimated that the average graft volume was 39 mm3, ranging from 10 to 107 mm3. Roughly 16,000 to 500,000 cells—about a third of those implanted—expressed the enzyme tyrosine hydroxylase, a marker of dopamine production. According to periodic 18F-DOPA PET scans, dopamine uptake gradually increased over 21 months to about half the level found in normal monkeys. This was true even in monkeys with the smallest grafts, and these animals also showed a treatment benefit.
That the graft sizes varied so widely suggests more work needs to be done to select the most successful cell lines for each recipient, said Studer. The researchers report that the most robust grafts expressed the epidermal growth factor Dlk1, and they detected no serotoninergic, GABAergic, cholinergic, or glutamatergic cells among them. Even so, Studer said the high proportion of non-dopamine cells in the transplants need to be characterized.
Ole Isacson of Harvard University pointed out that the 16,000 cells needed for therapeutic benefit here match the number he found a few years ago in monkeys (Hallett et al., 2015). “A major technical achievement that enhances our ability to plan the clinical trials is that the number of surviving cells needed for therapeutic benefit is in the same range,” he told Alzforum. The authors conclude that for people, about 100,000 cells would be the minimum needed for a motor effect, according to the paper. “That helps FDA determine the reasonable dose,” said Isacson.
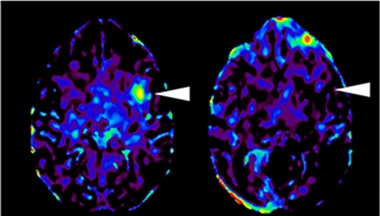
Troubling Mismatch.
On 11C-PK11195 PET scans, monkeys receiving a mismatched cell graft (left) had more activated microglia three months later than animals receiving cells with a matched MHC haplotype (right). [Courtesy of Takahashi et al., Nature Communications.]
A major concern with grafted cells is the potential for rejection. Implants derived from a patient’s own cells would be ideal, because they produce no immune response, but the process is lengthy and costly. As reported in the second paper, first author Asuka Morizane investigated a compromise—using immune compatible grafts. They matched the major histocompatibility complex (MHC) between donor and recipient monkeys to see if that would mitigate graft rejection. MHCs appear on cell surfaces and display proteins from inside the cell to watchful immune cells. They are crucial for those immune cells to distinguish “self” from “non-self.”
To do this, the authors derived two different iPSC lines from monkeys, turned them into a range of dopamine neural progenitor cells, and injected these cells into the left putamen of 16 monkeys: eight that had at least one identical MHC haplotype, and eight that were mismatched completely. Two animals from each group received the immunosuppressive drug tacrolimus to determine if that helped further. After three months, monkeys receiving the matched grafts did mount an immune response, but it was half as strong as that of monkeys receiving mismatched grafts. MHC matching worked about as well as giving tacrolimus along with the mismatched grafts. At four months, fewer activated microglia and infiltrating leukocytes were seen in the brains of matched monkeys and more dopaminergic neurons survived.
The study suggests that MHC matching gives dopamine cells an advantage, though immunosuppressants may still be necessary, wrote Takahashi to Alzforum. He will use MHC-homologous iPSCs in his clinical trial. Banking 50 MHC-characterized iPSC lines would largely cover the Japanese population, though the more heterogeneous populations of the United States and Europe would require hundreds of lines (Nakatsuji et al., 2008; Taylor et al., 2012).
“To have an HLA match for every patient is feasible, but requires steep cost and effort and doesn’t work perfectly,” said Studer. He took from the paper that tacrolimus suppresses the immune system well. For his part, Studer is planning a trial without MHC matching, developing one line of dopaminergic neurons and giving them to all patients along with immunosuppressants. Since matching still causes a minor immune response that would need to be suppressed anyway, he advocates for this cheaper approach.
Isacson took a different stance. Any immune reaction or requirement for immune suppression could stress an aged person’s system, he said. Since MHC matching doesn’t get rid of inflammation altogether, he saw it as evidence that iPSCs derived from a patient’s own cells will work best. They would require no immune suppression. Isacson acknowledged that deriving dopaminergic neurons from donor cells is expensive and hard to industrialize, but expects the cost will fall as the technology develops.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Barker RA, Barrett J, Mason SL, Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013 Jan;12(1):84-91. PubMed.
- Trounson A. Potential Pitfall of Pluripotent Stem Cells. N Engl J Med. 2017 Aug 3;377(5):490-491. PubMed.
- Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, Schumacher JM, Spealman RD, Isacson O. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell. 2015 Mar 5;16(3):269-74. Epub 2015 Feb 26 PubMed.
- Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008 Jul;26(7):739-40. PubMed.
- Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012 Aug 3;11(2):147-52. PubMed.
Further Reading
Papers
- Takahashi J. Strategies for bringing stem cell-derived dopamine neurons to the clinic: The Kyoto trial. Prog Brain Res. 2017;230:213-226. Epub 2017 Jan 2 PubMed.
- Kirkeby A, Parmar M, Barker RA. Strategies for bringing stem cell-derived dopamine neurons to the clinic: A European approach (STEM-PD). Prog Brain Res. 2017;230:165-190. Epub 2017 Feb 7 PubMed.
- Natalwala A, Kunath T. Preparation, characterization, and banking of clinical-grade cells for neural transplantation: Scale up, fingerprinting, and genomic stability of stem cell lines. Prog Brain Res. 2017;230:133-150. Epub 2017 Apr 7 PubMed.
- Lindvall O. Treatment of Parkinson's disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci. 2015 Oct 19;370(1680):20140370. PubMed.
- Buttery PC, Barker RA. Treating Parkinson's disease in the 21st century: Can stem cell transplantation compete?. J Comp Neurol. 2014 Aug 15;522(12):2802-16. Epub 2014 Apr 4 PubMed.
Primary Papers
- Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017 Aug 30;548(7669):592-596. PubMed.
- Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, Itoh Y, Okita K, Yamasaki E, Doi D, Onoe H, Ogasawara K, Yamanaka S, Takahashi J. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017 Aug 30;8(1):385. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.