Smooth Muscle Cells, Not Pericytes, Control Brain Blood Flow
Quick Links
What controls brain blood flow? Researchers disagree on exactly which vascular cells are responsible for constricting or dilating brain blood vessels, and what size vessels are involved. Some studies have pointed at pericytes, the cells that line brain capillaries, as the primary determinants of cerebral blood flow, but the findings have been controversial. In the June 25 Neuron, researchers led by Jaime Grutzendler at Yale School of Medicine, New Haven, Connecticut, report that pericytes lack the contractile protein actin, and play no role in squeezing blood vessels. Grutzendler and colleagues used various mouse lines with fluorescently labeled vascular cells to study the dynamics of blood flow in awake animals. They concluded that flow is exclusively controlled by smooth muscle cells surrounding the larger arterioles, which make up less than 10 percent of the brain’s blood vessels. These cells also regulated blood flow increases in response to brain activity, which form the basis for functional MRI. The findings may help researchers target the correct cells and develop better ways to restore normal flow in brains damaged by stroke or vascular dementia, the authors suggest.
Other researchers praised the methodology and the careful characterization of vascular cells. “The study uses state-of-the-art technology. The imaging is better than anything that’s been done before,” Maiken Nedergaard at the University of Rochester Medical Center, New York, told Alzforum. Costantino Iadecola at Weill Cornell Medical College, New York, agreed. “This will be a landmark paper for defining pericyte and smooth muscle cell biology in the brain,” he predicted.
Both pericytes and smooth muscle cells surround blood vessels, but the former are found on the smallest vessels and look very different from smooth muscle cells. Pericytes possess a small cell body and long, thin processes that extend lengthwise down vessels. They have been implicated in several vascular processes, including development and maintenance of the blood-brain barrier (see Feb 2015 Webinar; Mar 2015 conference news). Some studies reported that pericytes constrict capillaries and thus control the majority of cerebral blood flow and prevent tissue reperfusion after stroke (see Yemisci et al., 2009; Hall et al., 2014). Other groups disagreed, maintaining that arterioles are the main vessels that constrict or dilate to determine blood flow (see Fernández-Klett et al., 2010; Vates et al., 2010; Kornfield and Newman, 2014).
One problem may be the way these cell types were determined, Iadecola noted. Many studies identified pericytes using markers such as NG2 and PDGFRβ, which are also made in smooth muscle cells, or by the size of the blood vessels they surrounded. In addition, many previous studies were done in slice cultures, where blood flow cannot be directly examined.
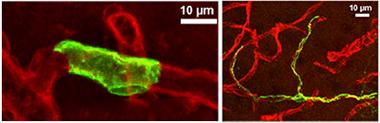
Rings vs. Tentacles:
Smooth muscle cells (left panel, green) form unbroken rings around blood vessels (red), while pericytes (right panel) have long, thin arms that stretch along capillaries for hundreds of microns. [Courtesy of Neuron, Hill et al.]
To get around these issues, Grutzendler and colleagues used transgenic mice. Joint first authors Robert Hill, Lei Tong, and Peng Yuan briefly turned on green fluorescent protein (GFP) under the control of the NG2 promoter in adult animals. This transient activation resulted in only a handful of smooth muscle cells and pericytes lighting up, allowing the researchers to distinguish single cells of each type. They found that smooth muscle cells, which form distinctive bands that wrap completely around vessels, appeared on arterioles that ranged in size from 3 to 40 microns in diameter. Pericytes, with their spidery processes, occurred on capillaries ranging from 3 to 9 microns in diameter, emphasizing that vessel size alone does not reliably distinguish these cell types (see image above).
The authors next characterized each cell type by whether it expressed the protein actin, which makes up the contractile filaments in muscle and other cells. In a mouse that expressed red fluorescent protein under the control of the actin promoter, only smooth muscle cells glowed red; pericytes did not. In the vascular tree, the authors saw a transition from actin-containing smooth muscle cells lining the larger vessels, to the occasional terminal smooth muscle cells dotting small precapillary arterioles, and finally to actin-free cells lining the fine vessels of the capillary bed (see image below). They also stained for actin in three postmortem human brains and saw a similar pattern. Overall, only about 8 percent of blood vessels in the human brain were surrounded by actin-containing cells.

Larger Vessels Control Contraction:
In mice, only smooth muscle cells surrounding arterioles express the contractile protein actin (red), while pericytes on capillaries (green) do not. (Green cells are astrocytes.) [Courtesy of Neuron, Hill et al.]
The authors tested the behavior of smooth muscle cells and pericytes in mice by expressing a calcium sensor in these cells as well as in neurons. Release of calcium into the cytosol correlates with depolarization and activation of both nerve and muscle cells. The authors found that spontaneous calcium ebbs and flows in smooth muscle cells correlated with arteriole dilation and contraction, respectively, in awake mice, whereas calcium levels in pericytes bore no relationship to capillary diameter. The authors then puffed air at the whiskers of the mice to stimulate the somatosensory cortex. In response, calcium levels rose in neurons and, after a delay of a second or two, dropped in smooth muscle cells. This drop correlated with a 10 percent dilation of the arterioles. Pericytes and capillaries remained unchanged. Taken together, the findings suggested that smooth muscle cells alone control increased blood flow in response to neural activity.
To test this idea more directly, the authors turned to a mouse that expressed the light-sensitive protein channelrhodopsin in smooth muscle cells and pericytes. When active, channelrhodopsin allows cations such as calcium to enter the cell. By shining light into a small patch of cortex, the authors activated both vascular cell types. In response, smooth muscle cells constricted vessels by 12 to 20 percent, but pericytes did not change capillary size.
All told, the experiments indicated that pericytes in the brain possess neither the machinery nor the ability to alter vessel diameter and control blood flow, Grutzendler told Alzforum.
Several commentators found the data compelling. “This is strong and convincing evidence,” Iadecola said. Roy Weller and Roxana Carare at the University of Southampton, U.K., found it thought-provoking. “The study shows that smooth-muscle actin is not a valid marker for pericytes, and that pericytes are not involved in active vasomotor responses as previously thought,” they wrote (see full comment below).
However, David Attwell at University College London objected to the way pericytes were defined in this study. The actin-containing cells that surround small precapillary arterioles look more like pericytes than smooth muscle cells, he wrote to Alzforum. “The data in this paper are entirely consistent with the work of Hall et al. and Yemisci et al., who showed that actin-containing capillary pericytes (erroneously mislabeled here as ‘smooth muscle cells’) regulate cerebral blood flow both in health and in disease. Given the overall similarity of the results, despite the vocabulary used, it would be a pity if the field were to become dominated by arguments over cells' names,” he wrote (see full comment below).
Grutzendler disagreed that this is a semantics issue. The cells on precapillary arterioles have a typical smooth muscle cell shape, with circumferential bands wrapping the vessel, he noted. “Utilizing a loose definition of pericytes as any mural cell that surrounds a brain microvessel (including first and second order branches of penetrating arterioles), regardless of morphology, molecular characteristics, or function, creates great confusion in the field,” he wrote to Alzforum.
Definitions aside, if the data hold up in future studies, what would that mean for diseases where blood flow is impaired? Grutzendler and colleagues investigated the role that smooth muscle cells might play in stroke by tying off the middle cerebral artery in transgenic mice for 90 minutes. They found that smooth muscle cells on precapillary arterioles clamped down on vessels in response, blocking the flow of red blood cells to downstream tissues. In many cases, the vessels remained constricted after the researchers removed the ligature and restored arterial blood flow. During occlusion, downstream capillaries frequently developed clots or their walls collapsed, preventing future blood flow (see Jul 2010 news) The findings help shed light on the “no reflow” phenomenon observed after stroke, where some brain areas remain starved for oxygen even after the main blood clot dissolves, Grutzendler said.
In future work, he will further study what happens in stroke models, including why capillaries become blocked and how that might be prevented. He will also investigate how cerebral amyloid angiopathy, a feature of Alzheimer’s, affects pericytes and smooth muscle cells. Some evidence indicates that pericytes are lost in aging and AD (see May 2014 conference news).
The findings may also have implications for functional MRI. This technique uses the increase in the flow of oxygenated blood to detect activation of brain cells. If the arterioles control blood flow and the smaller, more dispersed capillaries play no role, it suggests that blood flow can be altered only over relatively large brain areas, about 200 to 300 microns in diameter, Grutzendler said. This might set a practical lower limit on the resolution of fMRI techniques, he speculated.—Madolyn Bowman Rogers
References
Webinar Citations
News Citations
- Systemic Inflammation: A Driver of Neurodegenerative Disease?
- Capillary Clearing—Novel Mechanism a Link to Vascular Dementia?
- Fluid Markers and Imaging Back Idea of Breached Blood-Brain Barrier
Paper Citations
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009 Sep;15(9):1031-7. Epub 2009 Aug 30 PubMed.
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014 Apr 3;508(7494):55-60. Epub 2014 Mar 26 PubMed.
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22290-5. Epub 2010 Dec 6 PubMed.
- Vates GE, Takano T, Zlokovic B, Nedergaard M. Pericyte constriction after stroke: the jury is still out. Nat Med. 2010 Sep;16(9):959; author reply 960. PubMed.
- Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci. 2014 Aug 20;34(34):11504-13. PubMed.
Further Reading
News
- Treating Midlife Hypertension Helps Preserve Cognition in Old Age
- Glymphatic Flow, Sleep, microRNA Are Frontiers in Alzheimer’s Research
- Fluid Markers and Imaging Back Idea of Breached Blood-Brain Barrier
- It’s Not All About You, Neurons. Glia, Blood, Arteries Shine at Symposium
- Can Arterial Health Predict Amyloid Deposition?
- Silent Vascular Disease May Hasten Dementia Progression
- Does Brain Hypoxia Help Kick Off Alzheimer’s Pathology?
Primary Papers
- Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron. 2015 Jun 23; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southampton School of Medicine
University of Southampton School of Medicine
This paper is based on observations gained from a combination of sophisticated and elegant methods using transgenic mice that express fluorescent markers in pericytes and smooth muscle cells, and built upon the previous work and expertise of Jaime Grutzendler in the design of such mice. The images showing the morphology of the cells associated with capillaries, arterioles, and venules are of exceptional quality. Traditionally, it was considered that NG2, smooth muscle actin (SMA), desmin, and platelet-derived growth factor-β were all markers for pericytes, but recent work has demonstrated that a variable amount of SMA is expressed by pericytes. The present study shows that SMA is not a valid marker for pericytes and they are not involved in active vasomotor responses as previously thought.
Furthermore, transient middle cerebral artery occlusion resulted in vasoconstrictive responses exclusively from smooth muscle cells and not pericytes. These findings are highly significant for diseases associated with hypoperfusion of the brain and altered vasomotion, such as Alzheimer’s disease and cerebral amyloid angiopathy (CAA). One of the key pathogenic mechanisms of the development of CAA is the failure of perivascular lymphatic clearance of interstitial fluid and Aβ along the basement membranes of capillaries and arteries in the brain, as a result of biophysical changes associated with age-related stiffening of arterial walls. The present study opens up new methods and hypotheses involving the role of pericytes and smooth muscle cells in the pathogenesis of CAA.
The images in this paper are beautiful. But the most remarkable thing about the paper is that, while it confirms earlier data by Hall et al., 2014, and Yemisci et al., 2009, showing that capillary pericytes regulate blood flow both physiologically and after stroke, it is written as if it does the opposite.
This is because the authors use highly unconventional definitions of smooth muscle cell and of pericytes to reach their conclusions. Normally, vascular smooth muscle is defined as a layer of cells forming adjacent rings around arterioles (as in Figure 1A of the paper), while capillary pericytes have spatially isolated cell bodies looking like bumps on a log at roughly 30 micron intervals along capillaries. The authors, however, define a "pericyte" as a cell on a capillary that lacks smooth-muscle actin (a contractile protein) in processes around the vessel (for background information, pericytes have been known, since the work of Nehls and Drenckhahn, 1991, to show more actin expression when on microvessels near arterioles than when on vessels in the middle of the capillary bed). Any cell with a pericyte morphology that does have actin in circumferential processes they define to be a "smooth muscle cell," despite the vast difference in morphology of these actin-containing pericytes from true smooth muscle cells. Compare the green arteriole smooth muscle cells in Figure 1A with the cells indicated by arrowheads in Figure 1E; the latter are defined by the authors as "smooth muscle cells" despite the obvious similarity in appearance to the pericytes that are shown with arrows in Figure 1E. This inevitably leads to the erroneous conclusion that "pericytes" cannot regulate vessel diameter.
In fact, the data in this paper seem to be entirely consistent with the work of Hall et al. and Yemisci et al., who showed that capillary pericytes—the ones with actin—regulate cerebral blood flow both in health and in disease. Given the overall similarity of the results, despite the vocabulary used, it would be a pity if the field were to become dominated by arguments over cells' names. I hope, therefore, that people working in the field will rapidly resolve the issue of what to call these cells, so that the science and therapies based on it can be advanced.
References:
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014 Apr 3;508(7494):55-60. Epub 2014 Mar 26 PubMed.
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009 Sep;15(9):1031-7. Epub 2009 Aug 30 PubMed.
Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991 Apr;113(1):147-54. PubMed.
Medical University of South Carolina
The study by Hill et al. is a beautiful illustration of how structure predicts function. As Dr. Attwell has pointed out, a primary controversy here is a difficulty in identifying cells that reside at the junction between arterioles and capillaries. We recently published an article that characterized mural cell morphologies at this junction, using the same inducible NG2-CreERTM driver line. In our study, however, we found mural cells, which would have been called either “precapillary arteriole smooth muscle cells” or “pericytes” by Hill et al., difficult to cleanly categorize into either group.
Specifically, we found two types of cells that appeared to possess both characteristics of pericytes and smooth muscle cells. On the arteriole side of this junction, there were slightly elongated circumferential cells that expressed smooth muscle actin, just like smooth muscle cells. However these cells also exhibited ovoid, “bump on a log” somata more characteristic of classic pericytes (compare Fig. 4K of Hill et al. with Fig. 7f of ref (1)). On the capillary side of the junction, we found very elongated cells also with ovoid somata, but processes that formed a meshwork around the entire vessel lumen, unlike the thin stringy processes of pericytes deeper in the capillary bed. Interestingly, these cells expressed low levels of smooth muscle actin suggesting a possible role in control of vessel diameter (Fig 7h-k of ref (1)). Lacking an agreed-upon name for these cells, we called the former “smooth muscle-pericyte hybrid cells” and the latter a “mesh pericyte."
With this cellular heterogeneity in mind, it is likely that some of the contractile cells called “pericytes” by Hall et al. (2) during in vivo studies and “precapillary arteriole smooth muscle cells” by Hill et al., were these cells of mixed phenotype. Future studies will need to zoom in on the function of mural cells at the arteriole-capillary junction, as they may be last gatekeepers for flow into the capillaries beds.
References:
(1) Hartmann et al. (2015), Neurophoton. 2(4), 041402. http://neurophotonics.spiedigitallibrary.org/article.aspx?articleid=2300459
(2) Hall et al. (2014) Nature. 508(7494):55-60.
Make a Comment
To make a comment you must login or register.