PET in PD: Grafts Expose Symptoms, Cancer Agent Treats Mice
Quick Links
Tracers used in positron emission tomography double as imaging tools and therapeutic agents in two new papers on treatment strategies for Parkinson’s disease. In the April 5 Science, researchers led by Paola Piccini of Imperial College London, U.K., use clinical tests and PET imaging to show that dopamine grafts give PD patients lasting motor improvement, yet fail to slow the disease’s non-motor symptoms. And in a paper posted online April 2 in the Journal of Experimental Medicine, Kevin Barnham, University of Melbourne, Australia, and colleagues report that a cancer imaging agent improves motor and cognitive functions in four different PD animal models by scavenging harmful nitrogen radicals.
Though thought of primarily as a movement disorder, advancing Parkinson’s can also afflict mood, sleep, and cognition in ways that are equally, if not more, distressing than the motor symptoms. In the first paper, first author Marios Politis and colleagues asked whether intrastriatal tissue grafts—which can curb motor deficits in PD patients (see Lindvall and Björklund, 2004)—could relieve non-motor difficulties as well. The researchers clinically assessed all symptoms in three young-onset PD patients who received dopamine-rich fetal tissue grafts 13 to 16 years earlier (Hagell et al., 1999; Brundin et al., 2000), and used PET imaging to correlate the clinical symptoms with neuronal function.
The news was mixed. On the one hand, the tissue grafts brought lasting motor improvement—enough for patients to go off L-dopa medication a few years after transplantation and still not need the drug when analyzed for the current study. However, the dopamine grafts did not help with non-motor symptoms. Though the transplant patients’ cognition stayed intact, they battled depression, sleep disturbances, and a host of gastrointestinal and other problems more so than age-matched controls.
In brain scans with the PET ligand 18F-dopa, dopamine levels proved to have been restored to normal not only in the striatum, where the graft was placed, but also in other parts of the basal ganglia. This was also the case in the hypothalamus, insula, prefrontal cortex, thalamus, and locus coeruleus. But when the researchers used another PET marker, 11C-DASB, to measure the function of serotonergic neurons in the transplant recipients, they saw substantial degeneration in the raphe nuclei and other areas innervated by such neurons—including the amygdala, hypothalamus, and prefrontal cortex (see image below). “As expected, PD continues to progress elsewhere, as indicated by the loss of serotonin transporter binding in the raphe,” commented Kenneth Marek of the Institute for Neurodegenerative Disorders in New Haven, Connecticut. In a similar vein, deep-brain stimulation—another therapeutic approach for Parkinson’s—relieves motor impairment in some patients but leaves them with troubling cognitive and behavioral symptoms (see ARF related news story).
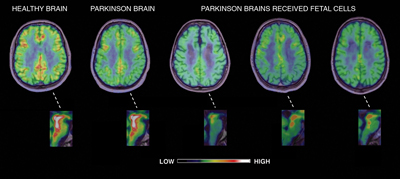
Parkinson’s disease patients receiving dopamine cell transplants show non-motor symptoms such as sleep, mood, and appetite disturbances, along with serotonin neuron loss in their brains. View larger image. Image credit: Marios Politis
Acknowledging that the current evidence is “circumstantial,” the authors propose that the low serotonin levels revealed by PET may underlie the non-motor symptoms of the grafted PD patients.
They suggest that additional grafts of serotonin cells in raphe nuclei or forebrain areas may help relieve these problems. This proposal “will be met with great skepticism,” David Grimes of Ottawa Hospital, Ontario, Canada, wrote in an e-mail to the Alzforum. “It is clear that a wide range of cells degenerate in more advanced PD. Targeting just one of these non-dopamine cell types is unlikely to reverse the many problems of advanced PD.” Moreover, Grimes noted, the data should be interpreted with caution, since the transplant patients were atypical in that they developed PD in their thirties and stayed free of dementia for 25 years of disease. (See full comment below.)
Further complicating matters, Politis and colleagues reported previously that contaminating serotonergic neurons contained in dopamine grafts can cause uncontrolled movements called dyskinesias as a side effect of transplantation (ARF related news story on Politis et al., 2010).
In the JEM paper, PET serves quite a different purpose. The Australian researchers stumbled on a PET tracer being developed for cancer imaging, and showed it was neuroprotective and improved behavior in four PD mouse models. The PET ligand, CuII(atsm), has a curious property. It hunts down the harmful nitrogen radical ONOO- (peroxynitrite) and inhibits its toxicity, as does uric acid, one of the body’s natural peroxynitrite scavengers. Epidemiological data suggest that people with high uric acid levels are protected against PD (Alonso et al., 2007; De Vera et al., 2008), whereas those with less uric acid are more susceptible to the disease (Hooper et al., 1998). Furthermore, ONOO- promotes nitration and aggregation of α-synuclein, and Lewy bodies have a lot of nitrated synuclein (Giasson et al., 2000). All this “indicates that nitration events are potentially quite important [in PD],” Barnham said.
First author Lin Hung and colleagues demonstrated in vitro that CuII(atsm) hastens ONOO- degradation, and blocks α-synuclein nitration and oligomerization. They then showed the compound could relieve stress caused by nitrogen free radicals in a neuroblastoma cell line. Finally, they took the approach into PD mouse models and found that oral administration of CuII(atsm) slowed neuronal death, reduced α-synuclein dimerization, rescued motor deficits, and improved memory. They showed the last two effects in hA53T mice overexpressing mutant human α-synuclein, and correlated these benefits with increased dopamine metabolite levels measured in Western blots, immunostaining, or PET imaging. To ensure the compound was truly neuroprotective and not just inhibiting the toxin’s actions in several of the PD models, the researchers were careful not to administer CuII(atsm) until the cell death cascade was well underway.
“This is probably the most rigorous preclinical evaluation of a compound that we’re capable of doing,” Barnham told Alzforum. Furthermore, when Japanese researchers used CuII(atsm) as an oxidative stress marker in a PET study of 15 PD patients (Ikawa et al., 2011), they reported that “the compound accumulates exactly where it’s supposed to—in the striatum,” Barnham said. “And the greater the disease severity, the more drug accumulated in that area.”
The compound looks promising for other neurodegenerative disorders as well. In a prior study (Soon et al., 2011), Barnham and colleagues found that CuII(atsm) extends lifespan and relieves motor deficits in a mouse model for amyotrophic lateral sclerosis (ALS). They are talking with potential commercial partners to advance the compound into clinical testing in PD or ALS. Meanwhile, the team is doing more mechanistic work and trying to design analogs, Barnham said.—Esther Landhuis
References
News Citations
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Serotonin Neurons—Culprits in Graft-Related Parkinson Dyskinesia
Paper Citations
- Lindvall O, Björklund A. Cell therapy in Parkinson's disease. NeuroRx. 2004 Oct;1(4):382-93. PubMed.
- Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, Widner H, Brundin P, Rothwell JC, Odin P, Wenning GK, Morrish P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Quinn NP, Lindvall O. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999 Jun;122 ( Pt 6):1121-32. PubMed.
- Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 2000 Jul;123 ( Pt 7):1380-90. PubMed.
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med. 2010 Jun 30;2(38):38ra46. PubMed.
- Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007 Oct 23;69(17):1696-700. PubMed.
- Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):675-80. PubMed.
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000 Nov 3;290(5493):985-9. PubMed.
- Ikawa M, Okazawa H, Kudo T, Kuriyama M, Fujibayashi Y, Yoneda M. Evaluation of striatal oxidative stress in patients with Parkinson's disease using [62Cu]ATSM PET. Nucl Med Biol. 2011 Oct;38(7):945-51. PubMed.
- Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, Tan JL, Lim NK, Lam L, Bica L, Lim S, Hickey JL, Morizzi J, Powell A, Finkelstein DI, Culvenor JG, Masters CL, Duce J, White AR, Barnham KJ, Li QX. Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem. 2011 Dec 23;286(51):44035-44. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med. 2010 Jun 30;2(38):38ra46. PubMed.
- Lindvall O, Björklund A. Cell therapy in Parkinson's disease. NeuroRx. 2004 Oct;1(4):382-93. PubMed.
- Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, Tan JL, Lim NK, Lam L, Bica L, Lim S, Hickey JL, Morizzi J, Powell A, Finkelstein DI, Culvenor JG, Masters CL, Duce J, White AR, Barnham KJ, Li QX. Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem. 2011 Dec 23;286(51):44035-44. PubMed.
Primary Papers
- Hung LW, Villemagne VL, Cheng L, Sherratt NA, Ayton S, White AR, Crouch PJ, Lim S, Leong SL, Wilkins S, George J, Roberts BR, Pham CL, Liu X, Chiu FC, Shackleford DM, Powell AK, Masters CL, Bush AI, O'Keefe G, Culvenor JG, Cappai R, Cherny RA, Donnelly PS, Hill AF, Finkelstein DI, Barnham KJ. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease. J Exp Med. 2012 Apr 9;209(4):837-54. PubMed.
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Björklund A, Lindvall O, Piccini P. Serotonin neuron loss and nonmotor symptoms continue in Parkinson's patients treated with dopamine grafts. Sci Transl Med. 2012 Apr 4;4(128):128ra41. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Ottawa Hospital
This is an impressive study in terms of the very long follow-up data in a highly selected group of three young-onset PD patients. Collecting detailed clinical and imaging data on patients after an intervention 13 to 16 years earlier is a significant task. It is remarkable that these three individuals continued to get improvement in their PD motor symptoms from the transplants after so many years and they continued not to require any levodopa medication. However, these individuals developed increasing difficulties with the non-motor features of PD.
We need to keep in mind these were very young patients whose disease started at ~34 years old. This is rare in PD. None of them had developed a dementia after more than 25 years of disease, which would not be typical for most PD individuals. This is often the most disabling non-motor feature of PD. Although the PD control group was matched for current age, the difference in disease duration between them was 20 years and so the comparisons made between the groups need to be interpreted with caution.
The authors try to link the non-motor symptoms of these three grafted PD individuals to their low serotonergic levels as measured by DASB PET scans. Their final comment that one might consider trying to transplant serotonergic cells to replace this deficit will be met with great skepticism by most researchers. It is clear that a wide range of cells degenerate in more advanced PD. Targeting just one of these non-dopamine cell types is unlikely to reverse the many problems of advanced PD.
We have a wide variety of treatment options that currently control the motor symptoms for most PD patients. Clearly we need to understand the overall degenerative process better before we can consider future dopaminergic AND non-dopaminergic transplantation techniques.
Banner Sun Health Research Institute
There is a tremendous need for agents that slow or stop progression of PD. This study of CuII(atsm) by Hung and colleagues does an admirable job of reviewing a variety of preclinical models of PD and demonstrating efficacy for this class of therapeutics. In addition to demonstrating benefits in animal models, it appears to have had restorative and anti-synuclein properties as well. No overt toxicity has been seen, although long-term follow-up has not been extensive. Short- and long-term primate studies are warranted, and then, hopefully, we will see studies in humans.
View all comments by Holly ShillPhiladelphia VA Medical Center
This is a very interesting study that provides many different lines of evidence to support a role for CuII(atsm) in blocking dopaminergic cell loss in several models of PD. Given the proposed mechanism of action regarding scavenging of peroxynitrate, it should not be surprising that this agent has efficacy in MPTP-mediated models of neurodegeneration. What was somewhat more surprising were the modest benefits seen in transgenic α-synuclein models of PD.
While there are still many questions to be answered, this report certainly suggests that this novel therapeutic target and agent deserve more attention as potentially neuroprotective in PD and other neurodegenerative conditions known to involve oxidative chemistry. Even though I was involved with some of the early studies examining nitration of α-synuclein, I was not convinced that nitrative modification of α-synuclein was far enough upstream in the pathophysiologic cascade to be a useful therapeutic target. This study makes a compelling argument that this may in fact be the case. I look forward to learning more about this compound and this avenue of investigation.
View all comments by John DudaMake a Comment
To make a comment you must login or register.