Not All Bad: Phosphorylated α-Synuclein Modulates Neurotransmission
Quick Links
Phosphorylation of α-synuclein at serine 129 has long been used as a marker of synucleinopathy—p-S129syn accumulates in Lewy bodies, after all. But does this modification have a physiological function? Two recent papers suggest that neuronal activity sparks S129 phosphorylation, which, in turn, drives synaptic vesicle trafficking. One paper suggests this facilitates neurotransmitter release but the other hints it may attenuate it. “This fascinating work helps shed light on the longstanding question of a neurophysiological function for p-S129syn,” wrote Michael Schlossmacher, University of Ottawa, Canada.
- Neural activity triggers phosphorylation of serine 129 in α-synuclein.
- p-S129syn binds presynaptic vesicle proteins and enables vesicle trafficking.
- Mice living in an enriched environment make lots of p-S129syn and have heightened synaptic plasticity.
In the January 16 NPJ Parkinson’s Disease, researchers led by Nagendran Ramalingam and Ulf Dettmer, Brigham and Women’s Hospital, Boston, reported that, in cultured cortical and hippocampal rat neurons, action potentials increase the amount of p-S129syn, which floods pre-synapses and increases excitatory signaling. In mice living in an enriched environment, namely in cages replete with novel objects to explore, p-S129syn filled neurons and synaptic plasticity was high. Environmental enrichment is known to boost plasticity, and learning and memory in mice (Sep 2005 news; Jankowsky et al., 2005).
In a bioRxiv preprint uploaded on December 23, last year, Subhojit Roy of the University of California, San Diego, and colleagues also reported a spike in presynaptic p-S129syn following neuronal activity. On a molecular level, p-S129 stabilized the C-terminus, allowing α-synuclein to bind synaptic vesicle proteins, they reported.
“If we are right and p-S129 is an ‘on’ switch for α-synuclein, then any past and future experiments have to be viewed through this lens—I think this is a major shift in thinking,” Roy told Alzforum. Michael Henderson of the Van Andel Institute, Michigan, agreed. “These studies are an important reminder to interpret antibody-dependent data carefully and that p-S129 staining alone is insufficient to establish the presence of Lewy pathology,” he wrote (comment below).
First author Ramalingam in Dettmer’s lab wondered if α-synuclein phosphorylation responded to neuron signaling. He used the GABA receptor antagonist picrotoxin (PTX) to dial up the firing of cultured rat cortical neurons. Six hours later, p-S129syn spiked about fourfold, while total α-synuclein did not change. On the other hand, suppressing neural activity with the sodium channel blocker tetrodotoxin (TTX) dropped p-S129syn by 25 percent. These results suggest that synaptic activity generates p-S129syn.
To see where this phosphorylation occurs, the researchers zoomed in on synapses using high-resolution confocal microscopy. They saw that p-S129syn foci overlapped with the presynaptic protein synapsin in both untreated and PTX-stimulated neurons. Synaptosomes isolated from those cells were enriched with the phosphorylated protein, hinting that p-S129syn was mostly within presynaptic vesicles.
How does neuronal activity lead to α-synuclein phosphorylation? A series of enzyme inhibition experiments confirmed that polo-like kinase 2 (Plk2) phosphorylates S129 and that protein phosphatase 2A (PP2A) dephosphorylates it, but also identified the calcium-dependent phosphatase calcineurin (CaN) as crucial for p-S129syn regulation (Inglis et al., 2009; Lee et al., 2011). The scientists believe that calcium released by action potentials activates CaN, which, in turn, increases Plk2 activity by some post-translational mechanism.
Does p-S129syn play any role in healthy neurons? Indeed, rat hippocampal neurons expressing α-synuclein with alanine at position 129, which cannot be phosphorylated, generated fewer excitatory action potentials and more inhibitory ones than did control neurons. Similarly, hippocampal slices from 1-month-old S129A mice could only muster weak short- and long-term potentiation (LTP). The authors concluded that phosphorylation of S129 stimulates synaptic firing. In vivo, environmental enrichment boosted p-S129syn in mice (see image below). Dettmer and colleagues think p-S129syn might alter plasticity by orchestrating synaptic vesicle recycling during neurotransmission.

Stimulating Synuclein. Wild-type mice living a cage with many toys (orange) had more p-S129syn (left) and enhanced long-term potentiation (right) than did animals in typical housing (purple). [Courtesy of Ramalingam et al., NPJ Parkinson’s Disease, 2023.]
Roy and colleagues found that S129 phosphorylation did just that. For their part, co-first authors Leonardo Parra-Rivas and Kayalvizhi Madhivanan overexpressed human α-synuclein in mouse hippocampal neurons. Wild-type protein tempered synaptic vesicle recycling, the phosphomimetic S129D suppressed it further, but the S129A had no effect, suggesting that S129 phosphorylation puts the brake on synaptic vesicle recycling. Roy thinks that this attenuates neurotransmitter release and, therefore, neural activity.
Like Dettmer and colleagues, researchers in Roy’s lab saw pre-synaptic p-S129syn rise, yet total α-synuclein remain steady, after stimulating cultured neurons. Ditto in wild-type mice. Adding TTX or a Plk2 inhibitor to cultured neurons prevented the uptick of p-S129syn. “It's reassuring that the Roy lab saw something similar to what we did,” Ramalingam said.
Parra-Rivas and Madhivanan also exclusively detected the S129D phosphomimetic in the pre-synapses, suggesting that phosphorylation encourages α-synuclein to flock there. What was p-S129syn up to in the pre-synapses? Phosphorylation facilitated α-synuclein’s binding to two synaptic partners involved in neurotransmitter release, synapsin, and VAMP2. S129D α-synuclein bound more of both proteins than did wild-type synuclein, while the S129A mutant bound neither. In mice stimulated with the potassium channel blocker 4-aminopyridine, α-synuclein also bound more synapsin and VAMP2-than it did in control animals.
To find out how phosphorylation facilitates binding, the scientists homed in on the C-terminus of α-synuclein, which is where S129 is located and VAMP2 and synapsin bind. They modeled the structures of wild-type and S129D α-synuclein using ColabFold, publicly available software that predicts three-dimensional protein structures (Mirdita et al., 2022). While the serine left the wild-type C-terminus unstructured, the negatively charged aspartic acid snuggled with five nearby positively charged lysine residues, causing the end of the protein to curl inward (see image below). The authors think this stabilizes the VAMP2/synapsin binding region, enabling the proteins to interact.
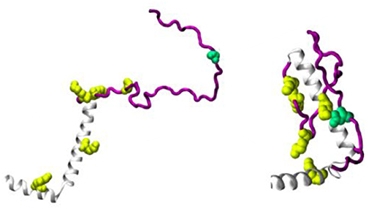
Opposites Attract. Molecular modeling suggests α-synuclein’s (gray) C-terminus (purple) remains unstructured when S129 (green) is unphosphorylated (left). The phosphomimetic S129D (right) attracts five positively charged lysine residues (yellow), folding into a configuration that binds VAMP2 and synapsin (not shown). [Courtesy of Parra-Rivas et al., bioRxiv, 2023.]
The distribution of p-S129syn throughout the mouse brain also caught Roy’s eye. He found it only in dopaminergic neurons of the midbrain and in olfactory neurons, regions susceptible to neurodegeneration in PD. Another recent bioRXiv preprint reported a similar pattern—p-S129syn accumulating within olfactory bulb neurons in healthy mice, rats, nonhuman primates, and people (Killinger et al., 2023). The phosphoprotein interacted with presynaptic vesicle trafficking and recycling proteins.
All told, the Roy and Dettmer labs found that phosphorylation of α-synuclein drives synaptic protein-protein interactions and facilitates neurotransmitter release, extending the phosphoprotein’s known function beyond just being a sign of synucleinopathy. “These exciting results challenge a central dogma in PD and open up new opportunities to decipher this pathology through the lens of synaptic physiology,” wrote Parra-Rivas and colleagues.—Chelsea Weidman Burke
References
News Citations
Paper Citations
- Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005 May 25;25(21):5217-24. PubMed.
- Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schöbel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009 Jan 30;284(5):2598-602. PubMed.
- Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X, Ray N, Fernandez JR, Chao Y, Masliah E, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM. Enhanced phosphatase activity attenuates α-Synucleinopathy in a mouse model. J Neurosci. 2011 May 11;31(19):6963-71. PubMed.
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods. 2022 Jun;19(6):679-682. Epub 2022 May 30 PubMed.
- Killinger BA, Mercado G, Choi S, Tittle T, Chu Y, Brundin P, Kordower JH. Distribution of phosphorylated alpha-synuclein in non-diseased brain implicates olfactory bulb mitral cells in synucleinopathy pathogenesis. bioRxiv. January 6, 2023 bioRxiv
Further Reading
Primary Papers
- Ramalingam N, Jin SX, Moors TE, Fonseca-Ornelas L, Shimanaka K, Lei S, Cam HP, Watson AH, Brontesi L, Ding L, Hacibaloglu DY, Jiang H, Choi SJ, Kanter E, Liu L, Bartels T, Nuber S, Sulzer D, Mosharov EV, Chen WV, Li S, Selkoe DJ, Dettmer U. Dynamic physiological α-synuclein S129 phosphorylation is driven by neuronal activity. NPJ Parkinsons Dis. 2023 Jan 16;9(1):4. PubMed.
- Parra-Rivas LA, Madhivanan K, Wang L, Boyer NP, Prakashchand DD, Aulston BD, Pizzo DP, Branes-Guerrero K, Tang Y, Das U, Scott DA, Rangamani P, Roy S. Serine-129 phosphorylation of α-synuclein is a trigger for physiologic protein-protein interactions and synaptic function. bioRxiv 2022.12.22.521485 bioRxiv
Annotate
To make an annotation you must Login or Register.

Comments
Van Andel Institute
These two studies provide new and intriguing information about a physiological role for α-synuclein phosphorylation at S129. pS129 α-synuclein is best known as a marker for Lewy pathology in Parkinson’s disease. However, a small portion of α-synuclein is phosphorylated under physiological conditions and the two teams cited here sought to determine what the role of that phosphorylated α-synuclein may be. In some ways, the two stories are remarkably similar. Both teams found that pS129 α-synuclein is present at low levels in the rodent brain and in primary neuron cultures, and that stimulation of neuronal activity increases the pool of pS129 α-synuclein. Phosphorylated α-synuclein seems to have an increased localization to the presynaptic terminal, where it has an effect on synaptic function. The two stories diverge on what this function is.
Parra-Rivas et al. use fluorescent live imaging to track synaptic vesicle dynamics and find that α-synuclein attenuates activity-dependent synaptic vesicle cycling, and that phosphorylation of α-synuclein exacerbates this effect. Ramalingam et al. use patch clamp electrophysiology and find that α-synuclein phosphorylation seems to promote neuronal activity through a feed-forward mechanism. It should be noted that both sets of experiments are largely supported by overexpression of phospho-mimetic or phospho-null mutant α-synuclein. It seems that future experiments will be necessary to determine the impact of pS129 α-synuclein on synaptic physiology.
How does activity stimulate α-synuclein phosphorylation? Ramalingam et al. provide convincing evidence that neuronal activity can stimulate PlK2 phosphorylation of α-synuclein through a calcium-calcineurin-dependent pathway. Parra-Rivas show via immunohistochemistry, that select neuronal populations appear to express elevated pS129 α-synuclein, while others express non-phosphorylated α-synuclein. It will be interesting to investigate what those neuronal populations are that express the highest level of physiological pS129 α-synuclein. Are they highly active neurons? Are they more susceptible to developing Lewy pathology?
These studies are also an important reminder to interpret antibody-dependent data carefully. For example, pS129 staining alone is insufficient to establish the presence of Lewy pathology. Non-pathological controls must stain negatively for positive staining to be interpreted as pathological. This control is especially important in transgenic mice that overexpress α-synuclein because the pool of soluble phosphorylated α-synuclein is likely much higher in these mice. The two studies presented here used a variety of pS129 α-synuclein antibodies to establish the noted phenotypes, and the congruent findings by both groups support the reproducibility of the findings. However, phospho-selective antibodies often have nonspecific staining, and this topic was revisited for α-synuclein antibodies in a recent publication (Lashuel et al., 2022).
The function of α-synuclein has been difficult to pin down over the years. Is this because its function is dynamically regulated via phosphorylation? Is it the same or a different pathway that regulates phosphorylation of pathological α-synuclein? Another aggregation-prone protein, tau, is physiologically phosphorylated, and there is some evidence that this phosphorylation could precipitate further pathological phosphorylation and misfolding. It will be interesting to see if α-synuclein phosphorylation fits a similar mold.
References:
Lashuel HA, Mahul-Mellier AL, Novello S, Hegde RN, Jasiqi Y, Altay MF, Donzelli S, DeGuire SM, Burai R, Magalhães P, Chiki A, Ricci J, Boussouf M, Sadek A, Stoops E, Iseli C, Guex N. Revisiting the specificity and ability of phospho-S129 antibodies to capture alpha-synuclein biochemical and pathological diversity. NPJ Parkinsons Dis. 2022 Oct 20;8(1):136. PubMed.
University of Tokyo
The University of Tokyo
The novel findings presented in the two papers, which suggest that normal α-synuclein has functions under physiological conditions, are quite interesting and unexpected. We had reported in 2002 that aggregated α-synuclein, but not the normal soluble species, is exclusively and highly phosphorylated at Ser129 (Fujiwara et al., 2002). Our estimation was that only ~4 percent of soluble α-synuclein was Ser129-phosphorylated, prompting us to speculate that the effect of α-synuclein phosphorylation in the normal state might be negligible. Since then, a number of studies using the Ser129Ala “unphosphorylatable” mutant have been conducted in variable contexts (e.g., Kuwahara et al., 2012), although the studies were mostly focused on toxicity, membrane binding, or aggregation properties of S129A mutant α-synuclein under an overexpression paradigm aiming at mimicking PD in model organisms.
The data presented in the two papers show that Ser129 phosphorylation of α-synuclein at physiological level is indeed increased in a neuronal activity-dependent manner. Furthermore, detailed analyses of the S129A mutant implicated the role of this phosphorylation in neuronal plasticity and/or synaptic vesicle transport in neurons. It is notable that the loss of Ser129 phosphorylation, which normally occurs in a few percent of molecules, elicited these effects, which may indicate that the phosphorylation leads to an acquisition of function, not an inactivation of constitutive activities. Of course, the results using the S129A mutant are always not free from the concern that the Ala substitution might have artifactually caused misconformation of α-synuclein, not a simple loss of phosphorylation.
The results of these studies further arouse our interest in the following points. First, is the regulatory mechanism(s) of α-synuclein phosphorylation in soluble state distinct from that of insoluble α-synuclein in synucleinopathy brains? Second, given our previous finding that soluble α-synuclein is extracellularly released in a neuronal activity-dependent manner in vitro and in vivo (Yamada and Iwatsubo, 2018), does the phosphorylation at Ser129 also affect the release of soluble α-synuclein? These questions may be of further interest in terms of the relevance of α-synuclein phosphorylation in disease and synaptic functions.
References:
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002 Feb;4(2):160-4. PubMed.
Kuwahara T, Tonegawa R, Ito G, Mitani S, Iwatsubo T. Phosphorylation of α-Synuclein Protein at Ser-129 Reduces Neuronal Dysfunction by Lowering Its Membrane Binding Property in Caenorhabditis elegans. J Biol Chem. 2012 Mar 2;287(10):7098-109. PubMed.
Yamada K, Iwatsubo T. Extracellular α-synuclein levels are regulated by neuronal activity. Mol Neurodegener. 2018 Feb 22;13(1):9. PubMed.
Make a Comment
To make a comment you must login or register.