Nano-size Quantum Dots Bust Up Synuclein Pathology
Quick Links
Best known to biologists as brightly glowing florescent tracers, quantum dots could also have therapeutic powers, says a new study. Work led by Han Seok Ko of Johns Hopkins University in Baltimore and Byung Hee Hong at the University of Seoul, South Korea, reveals that nano-scale carbon lattices bind to and dissolve α-synuclein fibers in vitro. In cells, the graphene quantum dots protect against α-synuclein toxicity, and prevent its spread between neurons. In animals, the dots protect neurons from the ravages of injected synuclein fibrils, or mutant synuclein expression, all without signs of toxicity. The findings are reported in the July 9 Nature Nanotechnology.
- Quantum dots made from graphene disaggregate α-synuclein fibers in vitro.
- In animals, the dots prevent α-synucleinopathy and improve motor function.
- Nanomaterials could provide new approaches to neuroprotection.
“This exciting study presents a novel and very untraditional way of treating synucleinopathy,” said Poul Henning Jensen, Aarhus University in Denmark. “They have done a good job of documenting their observation of a protective effect, but understanding the mechanism will take more work,” he said.
Quantum dots can be made of many materials—those derived from graphene (GQDs) consist of a single layer of carbon atoms arranged in a hexagonal lattice, sometimes with chemical modifications. GQDs look like small scraps of chicken wire, and are being developed as biosensors, imaging agents, and carriers for drug delivery. Interest in these nanostructures as therapeutic agents is on the rise. Some reports suggest they interfere with amyloid fiber formation, and disaggregate Aβ or α-synuclein fibrils (Mahmoudi et al., 2012; Li et al., 2014; Liu et al., 2015; Yang et al., 2015; Liu et al., 2018).

Plane of Attack.
In a molecular simulation, carboxyl groups (red) on a graphene quantum dot (gray lattice) bind to the amyloid core of α-synuclein fibril (tan), and unfold it via hydrophobic interactions. [Courtesy of Kim et al., 2018 Nature Nanotechnology.]
In the new study, co-first authors Donghoon Kim and Je Min Yoo tested the ability of chemically modified GQDs to counteract the aggregation, transmission, and toxicity of α-synuclein. By treating pure carbon fibers with strong acid, the investigators generated 2 nm square snippets of graphene that sport carboxylic acid groups around their edges. When mixed in equal amounts with purified α-synuclein in vitro, these GQDs potently inhibited fibril formation. Even more, they rapidly dissolved preformed fibrils, first melting the structures into shorter fragments and then into monomers. Structural analysis with NMR and other techniques indicated that the GQDs’ negatively charged carboxyl groups bound to the positively charged N-terminal region of α-synuclein in fibrils. A molecular dynamics simulation predicted that hydrophobic interactions between valine residues in α-synuclein and the GQD lattice destroy the β-sheet structure, driving fibril dissociation (see image at right). In agreement with that idea, removing the carboxyl groups destroyed the GQDs’ fibril-busting activity.
Moving to cell-based assays, the scientists demonstrated that GQDs protected primary cortical neurons from α-synuclein toxicity. The dots prevented cell death, restored neurite outgrowth and synaptic proteins, and mitigated mitochondrial toxicity induced by α-synuclein preformed fibrils (PFFs). Quantum dots also reduced the accumulation of phosphorylated and aggregated α-synuclein in response to PFFs, and blocked the transmission of α-synuclein pathology from cell to cell in vitro. Protection appeared to occur inside cells: In live imaging experiments, PFFs and GQDs were spotted together in lysosomes. There, the signal for PFFs decreased and that for GQDs increased over time, suggesting disaggregation of α-syn takes place in that compartment.
The protective effects of GQDs extended to two different PD mouse models. In one, the researchers injected α-syn PFFs into the striata of wild-type mice. After 180 days, six mice lost about half of their dopamine neurons, whereas six animals also given GQD injections twice a week lost only about one-quarter. Treatment also reduced the spread of gliosis, pathologic phosphorylated α-synuclein, and Lewy body-like inclusions (see image below). The animals’ motor function improved too. GQD-treated mice had more control of their paws and balanced better on a pole. In a transgenic PD model, pathology and motor function improved when mice expressing A53T mutated human α-synuclein were treated with the GQDs.
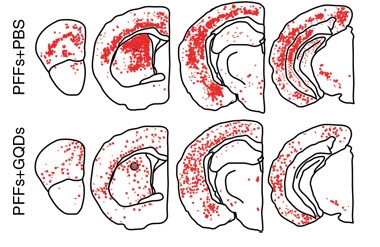
Nano Defense.
In wild-type mice, the spread of α-synuclein fibrils caused by striatal injection of PFFs (top) is mitigated by GQDs (bottom). [Courtesy of Kim et al., 2018 Nature Nanotechnology.]
The graphene dots even appeared safe. After four months of dosing, they caused no apparent harm to the mice. They were cleared in the urine. Multiple carboxyl groups packed onto teeny, inert scaffolds provide a potent, nontoxic, anti-aggregation agent, the authors concluded.
How did GQDs get into the brain? The dots readily traversed layers of endothelial cells and astrocytes in an in vitro model of the blood-brain barrier. Fluorescent imaging showed both cell types accumulated GQDs in lysosomes and released them via exosomes. The scientists propose that GQDs are endocytosed by endothelial cells, then released via exosomes, to be taken up by astrocytes, and finally released into the brain. In vivo, the researchers detected GQDs widely in the central nervous system after intraperitoneal injection.
Given studies suggesting they disassemble Aβ fibrils, these quantum dots might have applications beyond tackling synuclein fibrillogenesis. Ko told Alzforum they have confirmed that their GQDs can dissociate Aβ fibrils and oligomers in vitro. They are now testing the dots in a mouse model of AD, he said. At the same time, they are moving forward on the Parkinson’s disease front. Hong is working on safety studies in animals as a prelude to human use. “If all goes well we hope to start trials in people within two years,” Ko said. He believes GQDs will have a universal effect on neurodegenerative disorders that involve accumulation of fibrillar aggregates.
“This paper opens a new research direction on chemical modified nanomaterial for anti-amyloid therapy,” wrote Mingdong Dong, Aarhus University, Denmark, in an email to Alzforum. Dong’s group is evaluating other nanomaterial as anti-amyloid agents (Wang et al., 2018).
Jensen agreed, adding that he hopes the authors will clearly explain the procedure for making the dots. “I’m sure this paper will stimulate at lot of other studies. Their data suggest that it is important to produce the quantum dots in a specific way, and I hope they will describe the method in detail. If there is a trick to the production, it is important to document that,” he said. Jensen also told Alzforum he’d like to see more quantitation of the interaction between GQDs and fibrils, including measures of affinity and specificity for other amyloids, including nonpathogenic, physiological forms.—Pat McCaffrey
References
Paper Citations
- Mahmoudi M, Akhavan O, Ghavami M, Rezaee F, Ghiasi SM. Graphene oxide strongly inhibits amyloid beta fibrillation. Nanoscale. 2012 Dec 7;4(23):7322-5. PubMed.
- Li Q, Liu L, Zhang S, Xu M, Wang X, Wang C, Besenbacher F, Dong M. Modulating aβ33-42 peptide assembly by graphene oxide. Chemistry. 2014 Jun 10;20(24):7236-40. Epub 2014 May 18 PubMed.
- Liu Y, Xu LP, Dai W, Dong H, Wen Y, Zhang X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale. 2015 Dec 7;7(45):19060-5. Epub 2015 Oct 30 PubMed.
- Yang Z, Ge C, Liu J, Chong Y, Gu Z, Jimenez-Cruz CA, Chai Z, Zhou R. Destruction of amyloid fibrils by graphene through penetration and extraction of peptides. Nanoscale. 2015 Nov 28;7(44):18725-37. Epub 2015 Oct 27 PubMed.
- Liu Y, Xu LP, Wang Q, Yang B, Zhang X. Synergistic Inhibitory Effect of GQDs-Tramiprosate Covalent Binding on Amyloid Aggregation. ACS Chem Neurosci. 2018 Apr 18;9(4):817-823. Epub 2018 Jan 5 PubMed.
- Wang J, Liu L, Ge D, Zhang H, Feng Y, Zhang Y, Chen M, Dong M. Differential Modulating Effect of MoS2 on Amyloid Peptide Assemblies. Chemistry. 2018 Mar 7;24(14):3397-3402. Epub 2018 Feb 6 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, Lee S, Kwon SH, Kim S, Park YJ, Kinoshita M, Lee YH, Shin S, Paik SR, Lee SJ, Lee S, Hong BH, Ko HS. Graphene quantum dots prevent α-synucleinopathy in Parkinson's disease. Nat Nanotechnol. 2018 Jul 9; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Brigham and Women's Hospital, Harvard Medical School
Through this study, Ko and his colleagues are helping to shed light on the critical role of graphene quantum dots on disaggregation of α-synuclein fibrils (which are one of the hallmarks of Parkinson disease). The excellent in vivo outcomes of these graphene quantum dots extend researchers’ commitment to applying this new finding to improve health care and reduce the huge social and clinically deteriorating effects of neurodegenerative diseases such as Parkinson’s and Alzheimer’s. The role of these graphene quantum dots should be tested in amyloid-β fibrils in Alzheimer’s disease. However, the scientific community should carefully monitor the possible side effects of such an approach, as it might cause complications. Disaggregation of Aβ fibrils may create highly-toxic Aβ oligomers.
EPFL/ND BioSciences
This is a very interesting study that shows the potential of using quantum dots to modulate α-synuclein aggregation and protect against α-synuclein-induced toxicity and pathology spreading. However, the data presented do not support the proposed mechanism of action, i.e., that graphene quantum dots (GQD) inhibit and induce “complete disassociation of the fibrils” into monomers.
The proposed mode of action of GQDs can only be evaluated and commented on here on the basis of the in vitro aggregation data, because the assays used to assess the relationship between GQD modulation of α-synuclein fibril formation, pathology spreading, and toxicity do not allow for direct and quantitative assessment of the different aggregation states of α-synuclein.
GQDs treatment inhibits α-synuclein fibrillization
The thioflavin T and turbidity data shown in Figure 1b-d demonstrate that coincubation with GQDs completely blocked α-synuclein fibrillization. The absence of oligomers in the transmission electron microscopy images after seven days would argue for a mode of action where the GQDs act by stabilizing the monomeric state of α-synuclein. If true, this would be very exciting as it is rare to find molecules that stabilize the large and disordered conformation of the α-synuclein monomers.
Given this striking effect, it is not clear why the authors did not investigate the mechanism of inhibiting monomer aggregation and focused mainly on the interaction of the GQDs with the fibrils and their effect on fibril disassembly. It would have been nice to see circular dichroism/NMR data and solution measurements (light scattering, sedimentation velocity, or size-exclusion chromatography) on the conformation and distribution of α-synuclein species in the presence of GQDs at different time points and after seven days in particular. Do the GQDs stabilize a non-amyloidogenic monomeric conformation of α-synuclein?
“GQDs treatment inhibits a-synuclein fibrillization and disaggregates mature fibrils to monomers”
Although the data presented in Figures 1 and supplementary Figures 3 and 4 show that GQDs alter the aggregation and length of α-synuclein fibrils, they do not provide any direct evidence for disaggregation and/or disassembly of the fibrils into monomers. This can only be established using quantitative solution-based measurements such as light scattering, sedimentation velocity, or size-exclusion chromatography.
Fibril disaggregation
The BN-PAGE gels show a time-dependent increase in α-synuclein monomers. However, one could still detect a substantial amount of α-synuclein aggregates in the stacking gel. This is not consistent with the claim that GQDs induce complete disaggregation of preformed fibrils into monomers after seven days. This is also evident in the CD spectrum of the α-synuclein fibrils after one week of treatment with GQDs, which shows a spectrum that is consistent with a structure that is rich in β-sheet and β-turns. Given that monomeric α-synuclein exists predominantly in a disordered conformation, one would have expected to see a shift to a random coli (disordered) spectrum. The CD spectra and BN-PAGE results (Figure 1K and Supplementary Figure 4C) are not consistent with the EM data shown in Figures 1d, supporting figures and 1g, which were used to support disaggregation to monomers and show complete disappearance of fibrils and the absence of substantial amount of oligomers after seven days of treatment with GQDs.
The reported effects of GQDs on preformed fibrils by TEM (supplementary Figure 4) are also not consistent with the data reported by atomic force microscopy (AFM). The AFM data show that the α-synuclein fibrils prior to treatment with GQDs exhibit a length distribution from 600–1200 nm and that the addition of GQDs induce their disassociation leading to the population of short fibrils of less than 200 nm and 100 nm after 12 and 24 hours, respectively. However, the TEM data of the preformed fibrils before treatment with QGDs for one hour (Supplementary Figure 4) show a distribution of fibril length similar to that observed in previous experiments only in the presence of GQDs. Furthermore, the fibril length distribution of the preformed fibrils before and after treatment with QGDs for one hour was virtually identical and ranged from ~ 20–100 nm, with only a minor population of fibrils (six–10 fibrils) ranging in length from 180–200 nm.
Together, these discrepancies underscore the critical importance of using solution-based methods, combined with imaging techniques, to quantitatively assess the distribution of α-synuclein species (monomers, oligomers, and fibrils) and to achieve a more accurate understanding of the mechanisms of action of anti-amyloidgenic agents.
On fibril-seeding assays
It would be nice to know if the authors assessed whether the GQDs influence the extent of uptake/internalization of the preformed α-synuclein fibrils in their neuronal assays.
Since the authors suggest that they plan to start clinical trials in people within the next two years, it is crucial that there is a collaborative effort to replicate and validate these findings and test the proposed model. We will be happy to offer our expertise and contribute to this effort.
Make a Comment
To make a comment you must login or register.