Methylated RNA: A New Player in Tau Toxicity?
Quick Links
Tau aggregates are known to bind proteins and RNAs. Could this be why tangles are toxic? Researchers led by Benjamin Wolozin at Boston University think so. In the August 27 Molecular Cell, they reported that the RNA-binding protein HNRNPA2B1 and its binding partner, N6-methyladenosine (m6A) RNA, got tangled up in tau oligomers within cultured neurons. The cells showed signs of stress, but they recovered after the scientists knocked down HNRNPA2B1. In mouse models of tauopathy, this RNA-binding protein and m6A RNA colocalized with tangles and, again, knocking down HNRNPA2B1 saved neurons from dying. The scientists also spotted this molecular triad in the brains of people who had had Alzheimer's disease. Compared to controls, neurons from late-stage AD tissue contained more m6A RNA.
- In neurons, tau pulls methylated RNA, RNA-binding proteins, into cytosol.
- Knocking down the RNA and RBPs quelled tangles in mouse brain.
- In AD brain, methyl-RNA amasses in tangles as disease worsens.
Wolozin and colleagues think mislocalization of m6A RNA and its binding protein may contribute to the toxicity of tau oligomers. Mauro Montalbano at the University of Texas, Galveston, agreed. “This is potentially a new mechanism of neurotoxicity,” he wrote to Alzforum (full comment below).
“This paper adds to our growing understanding that toxic forms of tau greatly impact RNA metabolism and the nuclear architecture,” Bess Frost, University of Texas Health San Antonio, told Alzforum. Nick Seyfried from Emory University in Atlanta considers the work important. “This research suggests that pathological tau doesn’t act alone—it cooperates with RNA and RNA-binding proteins to give the molecular changes we see in disease,” Seyfried said.
This is not the first report that tau pilfers proteins from the nucleus. Others have shown that tau snatches RNA-binding proteins and their transcripts and drags them into the cytosol (Apr 2021 news; July 2017 news). Are tau oligomers to blame for this behavior?
To find out, first author Lulu Jiang and colleagues created a modified version of four microtubule-binding repeat domain (4R) tau. They fused the protein with mCherry, a fluorescent protein that is easy to detect, and with a cryptochrome peptide that oligomerizes when exposed to light. After transducing cultured mouse cortical neurons with DNA encoding this chimera, the scientists shined light on the cells to prompt oligomerization. Twenty minutes in the limelight was enough to create stable oligomers. After 60 minutes, tau began to form fibrils that bound thioflavin-S added to the cultures. These fibrils seeded tangles in tau sensor cell assays.
Did these fibrils spur degeneration of cultured neurons? Indeed, after 60 minutes of light, dendrites shriveled and became distorted. The neurons boosted activity of their caspase-3 enzyme, a sign of programmed cell death. They released lactate dehydrogenase into the culture medium, a harbinger of degeneration.
What was going on within these stressed neurons? Jiang found that tau became phosphorylated at T181 and S262, as indicated by increased binding of antibodies AT270 and 12E8 within the cells. Immunoprecipitated tau fibrils contained the nuclear envelope protein lamin B2, and its receptor. The researchers took this as a sign that tau fibrils had disrupted the nuclear membrane (Dec 2017 conference news). The neurons also made fewer proteins, as measured by their reduced incorporation of the translation inhibitor puromycin.
To figure out what triggered these breakdowns, Jiang and colleagues assessed changes in protein interaction networks. They exposed the neuron cultures to 0, 20, or 60 minutes of light, then immunoprecipitated mCherry, pulling down the tangles and any other proteins bound to them. Mass spectrometry identified proteins that were more, or less, abundant within tau oligomers. After 20 minutes of light, the IP captured nuclear envelope proteins and those involved in RNA metabolism and RNA splicing. Proteins involved in cell death co-immunoprecipitated after an hour of light.

Perturbed Proteins. In cultured neurons exposed to light for 20 (left) or 60 minutes (right), tau oligomers captured many proteins. Some were enriched within the oligomers, e.g. the RNA-binding protein HNRNPA2B1. [Courtesy of Jiang et al., Molecular Cell, 2021.]
A handful of captured proteins appeared to be the most trapped in the oligomer complexes (see image above). Top of the list: heterogeneous nuclear ribonucleoprotein A2/B1, aka HNRNPA2B1. Proteomics studies had fingered HNRNPA2B1 as a hub for perturbations seen in postmortem AD brains (Rayaprolu et al., 2020). Mutations in the prion-like carboxyl-terminal of HNRNPA2B1 muck up alternative splicing, trigger cell death in cultured human motor neurons, and cause familial amyotrophic lateral sclerosis (Oct 2016 news ; Mar 2013 news; Nov 2014 conference news).
Normally, HNRNPA2B1 resides in the nucleus, where it binds and stabilizes RNAs such as N6-methyladenosine (m6A) RNA. Being the most abundant mRNA modification, this methylation controls mRNA metabolism. This caught Jiang’s eye. M6A RNA is upregulated in the APP/PS1 mouse brain, and the enzyme that adds the m6A modification accumulates with insoluble tau in postmortem AD brain tissue (Han et al., 2020; Huang et al., 2020).
Might HNRNPA2B1 and m6A RNA be caught together by tau fibrils? The scientists added fluorescent anti-HNRNPA2B1 and anti-m6A antibodies to the neurons and exposed them to light for 60 minutes. The protein and RNA colocalized with tau fibrils in the cytosol, hinting that HNRNPA2B1 may drag the m6A RNA along for the ride. To verify this, the scientists knocked down about half of the HNRNPA2B1 by adding small interfering RNA to the cultured neurons. Accordingly, tau oligomers held 40 percent less m6A RNA. Knocking down HNRNPA2B1 also reduced the amount of cleaved caspase-3 and DNA damage, hinting that this protein may play a role in tau’s toxicity and neurodegeneration.
These molecular shenanigans are triggered by what are, after all, artificial tau oligomers. Do they also occur in animals? Indeed, Jiang and colleagues found similar changes in the PS19/P301S model, when HNRNPA2B1 and lamin B2 co-immunoprecipitated with tangles from the brains of six-month-old mice. Three, six, and nine-month-old mice had increasing amounts of the m6A RNA within the tangles, as well.
Could knocking down HNRNPA2B1 protect the mice? The scientists injected shRNA against the HNRNPA2B1 transcript into the hippocampi of three-month-old mice. Two weeks later, they injected tau oligomers taken from other P301S mice to stir tangle formation and, three weeks after that, they examined brain slices. Compared to P301S controls, knockdowns had less m6A and less cleaved caspase-3 in the hippocampus, and fewer tau oligomers. The authors concluded that HNRNPA2B1 contributes to tau toxicity, and the triad may aid oligomer formation.
Shanya Jiang of the University of New Mexico, Albuquerque, wondered if knocking down HNRNPA2B1 in older P301S mice could reverse cognitive deficits without any side effects (see full comment below).
How about people, though? Are HNRNPA2B1 and m6A RNA found within human tangles? The researchers obtained postmortem temporal cortex tissue from four controls and 14 people who had had AD from the AD centers at BU and Emory. For each Braak stage they had samples from three people. The scientists labeled the tissue with antibodies against tau, HNRNPA2B1, and m6A.
In controls, HNRNPA2B1 and m6A were near the nucleus, but at later AD stages, HNRNPA2B1 and m6A RNA were in the cytosol, where they colocalized with tau (see image below. The strongest colocalization of the troika occurred in Braak stages II-IV. M6A was diffusely distributed throughout the cytoplasm in late-stage disease, hinting that it may stray in ways independent of HNRNPA2B1 and tau.
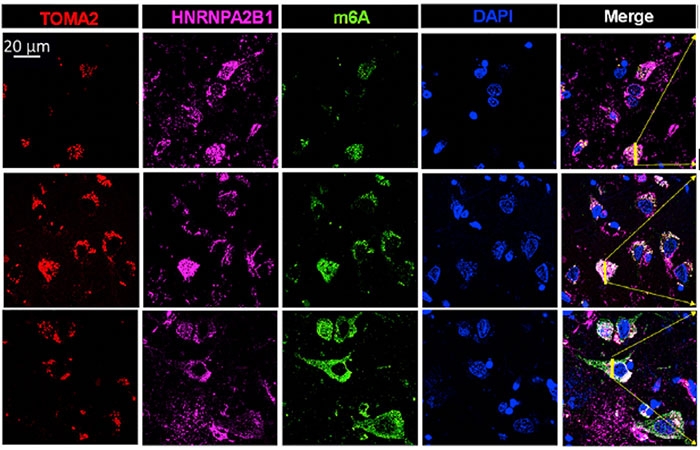
M6A Migration. Going from Braak stage I (top row) to stage III (middle) and VI (bottom row), more tau (red), HNRNPA2B1 (pink), and m6A (green) are found in the cytosol than at the nucleus (blue). [Courtesy of Jiang et al., Molecular Cell, 2021.]
The amount of methylated RNA seemed to be higher at later Braak stages, as well. Comparing Braak stages VI to I, Jiang found 4.5-fold more total m6A RNA isolated from brain tissue. Within tangles specifically, the scientists found six times more m6A in stage VI than in stage I. Similarly, they found that twice as many tau oligomers bound HNRNPA2B1 in frontal cortex tissue from Braak stage IV cases than in controls, despite cases and controls having similar amounts of total HNRNPA2B1. The authors chalked the differences up to the AD tissue having more tau oligomers and more HNRNPA2B1 in the cytosol than in the nucleus.
Given that methylated mRNAs seem to be important to tangles and toxicity, what exactly are these transcripts, and what do they do? That is not yet clear, but Wolozin’s group is sequencing the m6A transcripts from mouse and human brain to find out. Both Frost and Seyfried think it is important to know which m6A RNAs abound in the AD brain.
What about other tauopathies? Wolozin and colleagues are collecting brain tissue samples from people who had had ALS, frontotemporal dementia, or diffuse Lewy body disease, to quantify m6A RNAs in the neuronal cytosol and see if they co-localize with HNRNPA2B1 or tangles. The scientists are also studying the effects of the m6A methylation inhibitor STM2457 on tau oligomerization in brain organoids and APP knock-in/P301S mice (Yankova et al., 2021).—Chelsea Weidman Burke
References
News Citations
- Tau, Speckle Wrecker, Disrupts the Nuclear Home
- Tau Hooks Up with RNA to Form Droplets
- Is There No End to Tau’s Toxic Tricks?
- ALS Research ‘Gels’ as Studies Tie Disparate Genetic Factors Together
- Disease Mutations Zip Lock Stress Granules in Proteinopathy, ALS
- Stream of Genetics Pushes FTD Research Forward
Research Models Citations
Paper Citations
- Rayaprolu S, Higginbotham L, Bagchi P, Watson CM, Zhang T, Levey AI, Rangaraju S, Seyfried NT. Systems-based proteomics to resolve the biology of Alzheimer's disease beyond amyloid and tau. Neuropsychopharmacology. 2021 Jan;46(1):98-115. Epub 2020 Sep 8 PubMed.
- Han M, Liu Z, Xu Y, Liu X, Wang D, Li F, Wang Y, Bi J. Abnormality of m6A mRNA Methylation Is Involved in Alzheimer's Disease. Front Neurosci. 2020;14:98. Epub 2020 Feb 28 PubMed.
- Huang H, Camats-Perna J, Medeiros R, Anggono V, Widagdo J. Altered Expression of the m6A Methyltransferase METTL3 in Alzheimer's Disease. eNeuro. 2020 Sep/Oct;7(5) Print 2020 Sep/Oct PubMed.
- Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, Pilka ES, Aspris D, Leggate D, Hendrick AG, Webster NA, Andrews B, Fosbeary R, Guest P, Irigoyen N, Eleftheriou M, Gozdecka M, Dias JM, Bannister AJ, Vick B, Jeremias I, Vassiliou GS, Rausch O, Tzelepis K, Kouzarides T. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021 May;593(7860):597-601. Epub 2021 Apr 26 PubMed.
Further Reading
Papers
- Shafik AM, Zhang F, Guo Z, Dai Q, Pajdzik K, Li Y, Kang Y, Yao B, Wu H, He C, Allen EG, Duan R, Jin P. N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer's disease. Genome Biol. 2021 Jan 5;22(1):17. PubMed.
- Qin L, Min S, Shu L, Pan H, Zhong J, Guo J, Sun Q, Yan X, Chen C, Tang B, Xu Q. Genetic analysis of N6-methyladenosine modification genes in Parkinson's disease. Neurobiol Aging. 2020 Sep;93:143.e9-143.e13. Epub 2020 Apr 8 PubMed.
- Madugalle SU, Meyer K, Wang DO, Bredy TW. RNA N6-Methyladenosine and the Regulation of RNA Localization and Function in the Brain. Trends Neurosci. 2020 Dec;43(12):1011-1023. Epub 2020 Oct 8 PubMed.
Primary Papers
- Jiang L, Lin W, Zhang C, Ash PE, Verma M, Kwan J, van Vliet E, Yang Z, Cruz AL, Boudeau S, Maziuk BF, Lei S, Song J, Alvarez VE, Hovde S, Abisambra JF, Kuo MH, Kanaan N, Murray ME, Crary JF, Zhao J, Cheng JX, Petrucelli L, Li H, Emili A, Wolozin B. Interaction of tau with HNRNPA2B1 and N6-methyladenosine RNA mediates the progression of tauopathy. Mol Cell. 2021 Oct 21;81(20):4209-4227.e12. Epub 2021 Aug 27 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of New Mexico
This is an elegant study, which showed a novel mechanism of oligomeric tau causing neurotoxicity through inhibiting protein synthesis in neurons.
The authors optogenetically induced the expression of 4R1N wild-type (WT) tau in lentivirus-transduced primary cortical neurons in a time-dependent manner, which induced tau oligomerization and tau hyperphosphorylation at the T181 and S262 sites. Through proteomic profiling, the authors found that oTau can bind to HNRNPA2B1, a reader of m6A-modified transcripts, and can subsequently induce its nucleus-to-cytoplasm translocation.
HNRNPA2B1 linked oTau with m6A-modified mRNA in the cytoplasm. The oTau/m6A/HNRNPA2B1, which is increased five-fold in the individuals with AD, can further promote stress granule formation and nuclear envelop disruption, reduce protein synthesis, and induce apoptosis. Knocking down HNRNPA2B1 in vitro and in vivo both reduced oTau-induced neurodegeneration.
It will be important to see if knocking down HNRNPA2B1 in older P301S mice can rescue cognitive deficits without any side effects. It will be also important to see if tau oligomers made of 3R-tau have similar neurotoxic effects, which would be applicable to other tauopathies.
Additionally, it will be interesting to see if the oligomeric tau secreted from neurons and taken up by microglial cells exhibit the same neurotoxicity effect through interacting with m6A and HNRNPA2B1 and inhibiting protein synthesis in microglial cells.
University of Texas Medical Branch
The pathological “triade” composed of tau, RNA-binding proteins (RBPs) and RNA molecules strikes again. In recent years, laboratories around the world have published numerous studies on synergistic pathological function of RBPs and RNA in several neurodegenerative diseases. In neurons, toxic tau species, such as oligomers, catalyze the interactions with RBPs in several cell organelles and cellular locations.
In this well-designed study, the Wolozin lab added a new, exciting piece to the puzzle. Their combination of optogenetics (light-reactive bacterial cytochrome 2 – Cry2) and deep proteomic analysis identified HNRNPA2B1 as a principal RBP target of tau oligomer toxicity in stress granules. Moreover, they also observed that this hnRNP works as a molecular linker between tau oligomers and N6-methyladenosine (m6A) modified RNA transcripts. This reinforces growing evidence of synergy between tau and RBPs complexes, suggesting they mediate the progression of tauopathy.
Microtubule-associated protein tau stabilizes microtubules in healthy neurons. However, its aggregation state compromises its physiological functions. In Alzheimer’s disease and other tauopathies, tau hyperphosphorylation and accumulation induce neurotoxic effects at the cellular level, inducing biochemical and structural alterations. However, a knowledge gap in the biological functions of tau inducing stress and disease states still exists.
In this study, optically induced tau-Cry2 oligomers offered the ability to selectively induce tau oligomerization in a temporally controlled manner. This protein-protein interaction approach demonstrated a temporal evolution in tau interaction with different RBPs. The authors observed a perturbation of nuclear envelope components, confirming previous observations that support general RNA transport and metabolism impairment and defects.
Perhaps most importantly, this research points to involvement of m6A RNA transcripts in tauopathies and AD, representing a major finding in the field. The function of m6A is mainly studied in the cancer field, and is minimally investigated in neurodegeneration. This study is the first to link m6A to tau pathology, finding that m6A was transported to the cytoplasm under stress conditions and accumulated with tau oligomers upon tau aggregation induction.
Questions that remain to be addressed include the relative importance of tau oligomerization and phosphorylation, also how oligomerization of tau induces an nucleus-cytoplasm shuttling imbalance of RBPs and m6A transcripts. All these aspects are vital to uncovering the molecular mechanisms of RNA and protein nucleus/cytoplasm transport deficiency in AD and other tauopathies.
In summary, this elegant study demonstrates how tau oligomers function in stress conditions, presenting a new potential mechanism of neurotoxicity that may be suitable for developing new therapeutic strategies.
Make a Comment
To make a comment you must login or register.