Magnet Test Finds Cortex Overexcitable in All ALS
Quick Links
Scientists know that in people with some forms of amyotrophic lateral sclerosis, the motor cortex springs into action more often than it should. Now researchers conclude that the same occurs in people with ALS due to the C9ORF72 expansion, suggesting a phenomenon common to all forms of the disease. Writing in the September 8 JAMA Neurology online, they report that transcranial magnetic stimulation detects the same hyperexcitability pattern in people who inherited a C9ORF72 expansion as in those with sporadic ALS. The consistent hyperactivity of the motor cortex across ALS cases, often preceding noticeable movement symptoms, suggests this process could become both a diagnostic test and a therapeutic target, said senior author Steve Vucic of the University of Sydney.
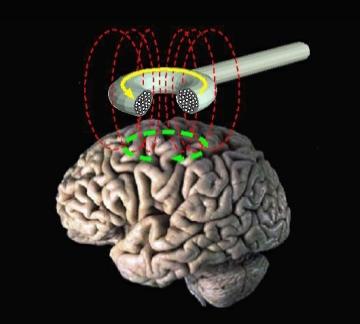
Brain On
Using an electromagnet to stimulate segments of the motor cortex, physicians measure how much current it takes to make specific muscles twitch. [Courtesy of Eric Wasserman, National Institute of Neurological Disorders and Stroke]
Vucic’s group studies how ALS affects the motor cortex, where, some scientists believe, the disease gets its first foothold (see Jan 2015 news; and news). Transcranial magnetic stimulation (TMS)—as a test, not a treatment—allows researchers to examine motor cortex function easily and painlessly in people. The researchers hold an electromagnetic coil against the scalp to stimulate the neurons that control a certain muscle, say, the thumb. Then they measure how much current it takes to make that thumb twitch. In several studies, Vucic and others noted that less current was needed to trigger movement in ALS patients, indicating that their motor cortex neurons were hyperexcitable (Vucic et al., 2013; see also Further Reading Papers, below). Vucic said he often detects hyperexcitability in people with an ALS mutation before they develop the disease. In sporadic cases, too, certain parts of the cortex become hyperexcitable before muscles downstream start to falter (Vucic et al., 2008).
While multiple research groups use TMS in this way, the increased hyperexcitability parallels other observable symptoms. For this reason, few clinicians have adopted TMS as a diagnostic test, said Alvaro Pascual-Leone of Beth Israel Deaconess Medical Center in Boston, who did not participate in the study. However, he said researchers have rekindled an interest in TMS because it may be useful in clinical trials. Researchers could use TMS as a quick test to check if drugs reduce excitability, and if patients benefit.
Vucic pointed out that in recent years, scientists have found cognitive difficulties as well as motor symptoms in many people with ALS (see Jul 2009 conference news). Indeed, the C9ORF72 expansion causes either ALS or frontotemporal dementia, or a mixture of both (see Sep 2011 news).
Therefore, first author Nimeshan Geevasinga and colleagues wondered if motor cortex excitability was heightened in people with C9ORF72 mutations as in sporadic cases. He found that cortex activity paralleled symptoms among 15 people with ALS due to the C9ORF72 expansion, 11 expansion carriers with no symptoms, 73 people with sporadic ALS, and 74 healthy controls. People with symptomatic ALS had hyperexcitability, regardless of their C9ORF72 status, but asymptomatic carriers activated movement,, as did controls. “Brain dysfunction in the form of hyperexcitability is an intrinsic feature of [symptomatic] ALS,” Vucic concluded.
The data indicate that simply possessing a C9ORF72 expansion is insufficient to change cortex excitability. This suggests to Vucic that other environmental factors contribute to disease onset.
The finding has important implications, commented Andrew Eisen of the University of British Columbia in Vancouver, who did not participate in the study. It means excitability would not make a useful marker for presymptomatic states of the disease, and that drugs to block hyperexcitability would not be helpful before symptoms begin, he said (see full comment below).
Despite those limitations, Vucic believes the commonality of hyperexcitability throughout ALS does qualify it as a useful diagnostic. He already uses TMS to confirm or rule out an ALS diagnosis in his practice; saying that 70 to 80 percent of people with ALS score high for hyperexcitability (Menon et al., 2015). TMS also helps him differentiate ALS from diseases that mimic its symptoms (Vucic and Kiernan, 2008; Vucic et al., 2011).
The researchers followed the asymptomatic C9ORF72 mutation carriers for up to three years during the study, and continue to do so, but none have developed ALS so far, Vucic said.
Vucic thinks researchers should target treatments to the brain where early signs of ALS arise. In a commentary accompanying the paper, Brian Wainger and Merit Cudkowicz of Massachusetts General Hospital in Boston agreed. “We believe the bulk of the evidence supports hyperexcitability as a primary process,” they wrote, “The concordance of the current study with prior findings warrants an intensified effort to identify how cortical and axonal hyperexcitability testing may yield new opportunities in ALS research and ultimately advance patient care.”
Wainger and colleagues will soon start a trial of retigabine, an anti-epileptic drug that blocks hyperexcitability in cultured neurons, with TMS scores as the primary outcome measure (Wainger et al., 2014).
Pascual-Leone suggested that TMS measurements are more objective than some clinical observations of ALS symptoms, and could serve as a biomarker to enable personalized medicine in the future. Based on a person’s degree of cortical excitability, physicians might determine the best treatment, and use TMS to follow whether the drug affects the cortex as desired, Pascual-Leone speculated.
However, researchers pointed out that questions about variability and consistency have been dogging TMS. Pascual-Leone said the field needs to know how TMS varies from day to day in the same individual. Vucic noted that factors such as sleep cycle or medication influence the TMS signal. Wainger and Cudkowicz called for more longitudinal studies on the timing of hyperexcitability compared to ALS onset and progression, and for animal studies to probe the mechanism behind that excitability.
Vucic plans to collaborate with researchers who study animal ALS models. He is conducting longitudinal studies that incorporate both TMS and MRI on people with ALS, including C9ORF72 carriers. MRI complements the excitability measurements TMS provides with a measure of cortical thinning associated with ALS.—Amber Dance
References
News Citations
- Earliest ALS Defects Said to Start in Disparate Places
- Are Upper Motor Neuron Gaffes a Prelude to Disease?
- London, Ontario: More Than Motor Malaise at ALS Meeting
- Corrupt Code: DNA Repeats Are Common Cause for ALS and FTD
Paper Citations
- Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry. 2013 Oct;84(10):1161-70. Epub 2012 Dec 21 PubMed.
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008 Jun;131(Pt 6):1540-50. Epub 2008 May 9 PubMed.
- Menon P, Geevasinga N, Yiannikas C, Howells J, Kiernan MC, Vucic S. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: a prospective study. Lancet Neurol. 2015 May;14(5):478-84. Epub 2015 Apr 3 PubMed.
- Vucic S, Kiernan MC. Cortical excitability testing distinguishes Kennedy's disease from amyotrophic lateral sclerosis. Clin Neurophysiol. 2008 May;119(5):1088-96. Epub 2008 Feb 29 PubMed.
- Vucic S, Cheah BC, Yiannikas C, Kiernan MC. Cortical excitability distinguishes ALS from mimic disorders. Clin Neurophysiol. 2011 Sep;122(9):1860-6. Epub 2011 Mar 5 PubMed.
- Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, Brown RH Jr, Cudkowicz ME, Bean BP, Eggan K, Woolf CJ. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014 Apr 10;7(1):1-11. Epub 2014 Apr 3 PubMed.
External Citations
Further Reading
Papers
- Chervyakov AV, Bakulin IS, Savitskaya NG, Arkhipov IV, Gavrilov AV, Zakharova MN, Piradov MA. Navigated transcranial magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve. 2015 Jan;51(1):125-31. Epub 2014 Nov 22 PubMed.
- Chase A. Motor neuron disease: Evaluation of ALS via transcranial magnetic stimulation. Nat Rev Neurol. 2014 Sep;10(9):485. Epub 2014 Aug 12 PubMed.
- Geevasinga N, Menon P, Yiannikas C, Kiernan MC, Vucic S. Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis. Eur J Neurol. 2014 Apr 2; PubMed.
- Inghilleri M, Iacovelli E. Clinical neurophysiology in ALS. Arch Ital Biol. 2011 Mar;149(1):57-63. PubMed.
- Attarian S, Pouget J, Schmied A. Changes in cortically induced inhibition in amyotrophic lateral sclerosis with time. Muscle Nerve. 2009 Mar;39(3):310-7. PubMed.
- Turner MR, Osei-Lah AD, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, Leigh PN, Mills KR. Abnormal cortical excitability in sporadic but not homozygous D90A SOD1 ALS. J Neurol Neurosurg Psychiatry. 2005 Sep;76(9):1279-85. PubMed.
News
Primary Papers
- Geevasinga N, Menon P, Nicholson GA, Ng K, Howells J, Kril JJ, Yiannikas C, Kiernan MC, Vucic S. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015 Nov;72(11):1268-74. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of British Columbia
Vucic and colleagues in Australia have, over the last decade, published extensively on hyperexcitability in ALS, and there can be little debate regarding the validity of the phenomenon. The sophisticated physiology used to investigate ALS-related hyperexcitability indicates that it reflects a combination of reduced inhibition from impaired GABA functioning and increased glutamate activity. The present study confirms the presence of cortical hyperexcitability in symptomatic patients with C9ORF72 familial ALS (FALS), as has been previously noted in ALS patients with other genetic mutations, suggesting that cortical hyperexcitability is of pathophysiological importance in ALS, as well as in sporadic ALS (SALS), irrespective of underlying genetic status.
However, cortical excitability is normal in asymptomatic FALS, independent of the type of mutation, so the development of hyperexcitability in FALS is a late feature, possibly mediating neuronal degeneration via a transsynaptic anterograde process. It is important that this latency has been established, because protective therapy aimed at preventing or reducing hyperexcitability in the presymptomatic period will likely be of no avail. Furthermore, detecting cortical hyperexcitability as a marker of presymptomatic FALS or SALS is not going to be useful. However, cortical hyperexcitability in ALS is an interesting phenomenon, and much still remains to be learned about it. It may turn out that reduced inhibition (GABA impairment) is the crucial element, which could be of value as an early, presymptomatic disease marker.
Chiba University
Chiba University
Motor Cortical Hyperexcitability and Neuronal Death in Familial and Sporadic Amyotrophic Lateral Sclerosis
Widespread fasciculations are a prominent clinical feature in patients with ALS, indicating abnormally increased excitability and spontaneous firing of lower motor neurons/axons. Fasciculations often arise from the motor nerve terminals, and partly from spinal motor neurons (de Carvalho et al., 2013). These findings suggest altered excitability occurs in both the distal motor axons and cell body of lower motor neurons.
Over the past 20 years, our understanding of the ionic pathogenesis for fasciculations has been expanded. Peripheral motor axonal excitability studies with a threshold-tracking technique have shown increased nodal persistent sodium currents and reduced potassium currents in ALS patients, both changes leading to increased axonal excitability, and thereby generation of fasciculations (Kanai et al., 2006).
Separately, a long and ongoing debate regarding the processes underlying motor neuronal death in ALS continues, and corticomotoneuronal hyperexcitability via an anterograde trans-synaptic glutaminergic process has been proposed as an underlying mechanism (Eisen et al., 1992). Support for this primary cortical involvement hypothesis has been provided by TMS studies establishing that cortical hyperexcitability was an early event of patients with sporadic ALS; TMS has shown hyperexcitability of the motor cortical circuitry (Vucic et al., 2013). Specifically, short-interval intracortical inhibition (SICI) with paired pulse TMS threshold tracking was significantly reduced in patients with sporadic ALS, and in those with familial ALS caused by mutations in the copper/zinc superoxide-dismutase-1 (SOD-1) gene. This reflects changes in intrinsic motor cortical circuits presumably mediated by altered actions in GABAergic neurotransmission (Vucic et al., 2008).
The present study nicely demonstrated this in asymptomatic carriers and patients with C9ORF72 ALS. The authors measured SICI with TMS threshold tracking, and found that the results largely paralleled clinical states; patients with C9ORF72 expansion had significantly reduced SICI, and motor cortical hyperexcitability very similar to people with sporadic ALS. In contrast, asymptomatic carriers showed normal SICI. These findings strongly suggest that the onset of ALS is closely associated with cortical hyperexcitability of the motor cortex. The disinhibition may be caused by loss of GABAergic inhibitory interneurons in the motor cortex. Further studies will be required to elucidate the mechanisms for the altered cortical function.
Another important finding is that markedly reduced SICI in C9ORF72 ALS patients is exactly the same as those observed in familial ALS patients with different genetic mutations such SOD-1 mutation (Vucic et al., 2008). This suggests that sporadic ALS and familial ALS with different genetic status share a common pathophysiological mechanism. For both sporadic and familial ALS, suppression of neuronal and axonal excitability of cortical, as well as spinal, motor neurons could be a neuro-protective strategy. Stabilization of the neuronal membrane with persistent sodium channel blockers or potassium channel openers will be therapeutic options for the total population of ALS.
References:
de Carvalho M, Swash M. Origin of fasciculations in amyotrophic lateral sclerosis and benign fasciculation syndrome. JAMA Neurol. 2013 Dec;70(12):1562-5. PubMed.
Kanai K, Kuwabara S, Misawa S, Tamura N, Ogawara K, Nakata M, Sawai S, Hattori T, Bostock H. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain. 2006 Apr;129(Pt 4):953-62. Epub 2006 Feb 8 PubMed.
Eisen A, Kim S, Pant B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron?. Muscle Nerve. 1992 Feb;15(2):219-24. PubMed.
Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry. 2013 Oct;84(10):1161-70. Epub 2012 Dec 21 PubMed.
Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008 Jun;131(Pt 6):1540-50. Epub 2008 May 9 PubMed.
Make a Comment
To make a comment you must login or register.