Molecular Transport Vehicle Shuttles Therapies into Brain
Quick Links
Researchers have devised a new way to sneak therapeutic cargo across the blood-brain barrier. Into the constant region of a human IgG1 antibody, they slid a domain that latches onto receptors that carry transferrin into the brain. On the other end of the IgG, the researchers attached therapeutic cargo, which the “transport vehicle” ferried across and released throughout the brain in both mice and monkeys. The strategy was reported in two papers published May 27 in Science Translational Medicine.
- Antibody Fc region engineered to bind the transferrin receptor.
- This “transport vehicle” carried BACE1 antibody, knocked down Aβ in mice and monkeys.
- Delivering an enzyme, it corrected a lysosomal storage disease in mice.
In one, Adam Silverman, Joy Zuchero, and colleagues at Denali in South San Francisco detailed the vehicle’s development, and showed that brain Aβ plummeted when animals were injected with the engineered IgG1 carrying two antibody Fab fragments that bind BACE1. In the second, Mihalis Kariolis, Anastasia Henry, and colleagues described how they used the vehicle to deliver iduronate 2-sulfatase into the brain. This enzyme is lacking in people with Hunter syndrome, a devastating lysosome-storage disease. In a mouse model of Hunter syndrome, the ETV, aka enzyme transport vehicle, restored normal lysosomal function while stemming neuroinflammation and axonal damage. In all, the findings suggest that the vehicle is capable of transporting myriad therapeutic cargo into the brain, including antibodies, enzymes, and potentially oligonucleotides.
Torben Moos of Aalborg University in Denmark called the approach highly promising. “I’m very optimistic that this approach will create major changes in our ability to deliver therapies into the brain,” he told Alzforum.
Berislav Zlokovic of the University of Southern California, Los Angeles, called the transport vehicle platform an important advance in the field of transferrin receptor (TfR) based approaches to cross the blood-brain barrier (BBB). However, he asked how a compromised BBB in neurological diseases, including Alzheimer’s, may affect the function of the TfR system.
The BBB is a formidable roadblock for therapeutics, especially for large ones such as antibodies or enzymes. To outsmart the barriers, scientists have attempted to co-opt receptor-mediated transcytosis pathways that transport essential cargo into the brain. The TfR is the most researched of these.
An early attempt tethered therapeutics to TfR antibodies (Pardridge, 2002). Alas, high-affinity versions of these antibodies tended to get snagged in the cerebrovasculature, stifling cargo delivery into the brain. Scientists at Genentech tried to get around this with bispecific antibodies, where one arm loosely bound TfR and the other latched onto a therapeutic target. This improved delivery (May 2011 news).
Roche (which acquired Genentech) took a modular approach with its Brain Shuttle (see video). It relies on a separate TfR-specific Fab fragment that can be attached to the constant region of a therapeutic antibody, or theoretically to other types of molecules (Jan 2014 news). Roche started a Phase 1 trial evaluating a shuttle version of the Aβ-immunotherapy gantenerumab in November 2019. Yet other configurations, such as a therapeutic antibody with TfR Fabs attached to its armpits, are also in the works (Hultqvist et al., 2017; Jan 2018 news).
Enter Denali’s BBB transport vehicle. As co-first authors Kariolis and Robert Wells described, it can carry all manner of cargo. Rather than attach an additional TfR-specific antibody fragment to the immunoglobulin Fc region, or waste a precious antibody arm targeting TfR, the researchers incorporated a slimmed-down, novel TfR-binding domain into the constant region of a human IgG1. They identified a nine-amino-acid stretch of the Fc stub that appeared to tolerate meddling, and randomized its amino acid sequence. Screening the mutant libraries in yeast, they pulled out a handful of constant region clones bestowed with a penchant for binding TfR. They purposefully selected one that cross-reacted with TfR from cynomolgus monkeys, allowing future studies in nonhuman primates.
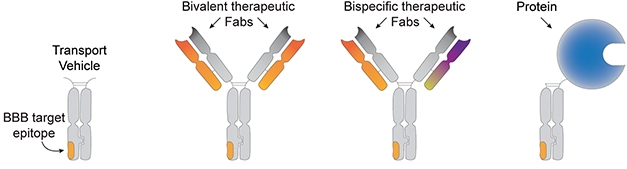
Mix and Match. The BBB transport vehicle (left) is an antibody Fc region harboring a domain that binds to the transferrin receptor. The TV can be affixed with antibody Fab regions specific for one or two epitopes (middle), or with other types of molecules, e.g., enzymes (right). [Courtesy of Kariolis et al., Science Translational Medicine, 2020.]
After testing transport vehicles in cell culture studies, the researchers stuck them into chimeric, human TfR knock-in mice. In those, a portion of human TfR that the transport vehicle recognizes is knocked into one copy of the mouse TfR gene. The scientists injected an antibody transport vehicle (ATV) carrying two Fab arms specific for BACE1 intravenously into these knock-in mice. This did not douse normal expression of TfR in the brain, even after chronic dosing. Compared with mice injected with regular BACE1 antibodies, those treated with ATV-BACE1 had 40 times more anti-BACE1 in their brains 24 hours later. Depending on the timepoint, ATV-BACE1 had a 10- to 100-fold greater brain-to-plasma ratio than did typical anti-BACE1 antibodies. ATV-BACE1 engaged its target, triggering double the drop in soluble brain Aβ40 as that seen in anti-BACE1 antibodies.

Busting into the Brain. Four hours after intravenous injection, ATV-BACE1 (pink) mingles in endothelial cells (white) of brain blood vessels (top). By 24 hours (bottom), ATV-BACE1 has found its way into the brain parenchyma, and even within neurons (blue). [Courtesy of Kariolis et al., Science Translational Medicine, 2020.]
Notably, while most of the ATV-BACE1 was spotted in the cerebrovasculature four hours after dosing, by 24 hours it had penetrated the brain parenchyma of the cortex, hippocampus, and cerebellum. Super-resolution confocal imaging of brain sections revealed that neurons had readily internalized ATV-BACE1. By contrast, anti-BACE1 antibodies made a sparse showing in both the vasculature and the parenchyma.
Finally, the researchers tested ATV-BACE1 in cynomolgus monkeys, which express TfR in their brain vasculature. They dosed the monkeys with ATV-BACE1, anti-BACE1, or a control IgG, then tracked various APP products. In animals treated once with ATV-BACE1, CSF concentrations of Aβ40 plummeted by 70 percent, while the ratio of APPβ/APPα, an indicator of BACE1 cleavage activity, dropped by 75 percent two days after dosing. By 14 days, levels had returned to baseline. CSF Aβ40 or APPβ/APPα did not drop in monkeys treated with anti-BACE1 or control IgG. In another experiment, the researchers found that two days after dosing, levels of ATV-BACE1 were 26- to 35-fold higher across multiple brain regions than levels of anti-BACE1 antibodies, suggesting that the transport vehicle significantly boosted delivery into the brain.
The BACE1-ATV studies were designed to test delivery and target engagement in the brain, Steve Krognes of Denali told Alzforum. In part due to disappointing results of BACE1 inhibitor trials, the company will not pursue BACE1 as a target in AD clinical trials, he said.
Instead, Denali set its sights on the microglial receptor TREM2, for which activating antibodies have been developed and fused to the transport vehicle (May 2019 conference news; Mar 2020 news). For amyotrophic lateral sclerosis and frontotemporal dementia, Denali is testing out a TV loaded with progranulin, a lysosomal protein that is dysfunctional in some people with those neurodegenerative diseases.
However, the cargo nearest to clinical development is one to treat Hunter syndrome. This childhood lysosomal-storage disease wreaks havoc throughout the body. In the second STM paper, first authors Julie Ullman and Annie Arguello reported that an ETV loaded with iduronate 2-sulfatase (IDS) crossed into the mouse brain and restored lysosomal function there.
IDS deficiency leads to a buildup of the enzyme’s substrates, namely glycosaminoglycans (GAGs), in the lysosome. This in turn hobbles lysosomal function, leading to an accumulation of myriad other lysosomal substrates that normally would be destroyed. The disease affects multiple organs, and in about two-thirds of patients, the brain. Intravenous injection of recombinant IDS is the standard of care; however, the recombinant enzyme bounces off the BBB and thus does nothing to stem neurological symptoms, which can include cognitive impairment.
To learn if a transport vehicle could deliver the enzyme to the brain, the scientists injected their ETV-IDS intravenously into human TfR knock-in mice that had their IDS knocked out. Just like recombinant IDS, ETV-IDS reduced GAGs in the spleen and liver. However, only ETV-IDS entered the brain and disposed of GAGs there, as well. This dramatically reduced the build-up of other lysosomal lipids and proteins, and dampened neuroinflammation, a hallmark of Hunter disease. The transported enzyme also lowered the concentration of neurofilament light (NfL) in the mouse CSF, suggesting that the treatment staunched neurodegeneration.

Lipid Clean-Up. These heat maps show how IDS knockout mice (left) have a build-up of two types of lysosomal lipid in the brain (top and bottom). Treatment with ETV-IDS (right) reduced accumulation, while vehicle (left) or IDS alone (middle) did not. [Courtesy of Ullman et al., Science Translational Medicine, 2020.]
“This is a cool tool that could be potentially applied to treat many lysosome-storage diseases as well as adult-onset neurodegenerative diseases,” commented Fenghua Hu of Cornell University in Ithaca, New York.
Paul Saftig of the University of Kiel in Germany agreed. “This is certainly a major step forward in the development of an effective therapy of Hunter syndrome (MPSII) and maybe other lysosomal-storage disorders,” he wrote. That said, Saftig offered a word of caution. Because the ETV-IDS fusion protein is unknown to the body and requires chronic dosing, it could rouse problematic immune reactions, including generation of antibodies that could erode the treatment’s efficacy (see comment below).
Denali will begin a Phase 1/2 clinical trial testing an ETV-IDS in children with Hunter syndrome. Beyond enzymes and therapeutic antibodies, the scientists are also working on attaching anti-sense oligonucleotides to the transport vehicles. “The strongest feature of this platform is its modularity,” Kariolis said.—Jessica Shugart
References
News Citations
- Smuggling Antibodies to BACE Across the Blood-Brain Barrier
- Brain Shuttle Ferries Antibodies Across the Blood-Brain Barrier
- Antibody Shuttle Rouses Anti-Aβ Response in Brain without Waking the Periphery
- Antibodies Against Microglial Receptors TREM2 and CD33 Head to Trials
- Paper Alert: Mouse TREM2 Antibody Boosts Microglial Plaque Clean-Up
Paper Citations
- Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002 Feb;1(2):131-9. PubMed.
- Hultqvist G, Syvänen S, Fang XT, Lannfelt L, Sehlin D. Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor. Theranostics. 2017;7(2):308-318. PubMed.
Other Citations
Further Reading
Primary Papers
- Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, Giese T, Assimon VA, Chen X, Zhang Y, Solanoy H, Jenkins K, Sanchez PE, Kane L, Miyamoto T, Chew KS, Pizzo ME, Liang N, Calvert ME, DeVos SL, Baskaran S, Hall S, Sweeney ZK, Thorne RG, Watts RJ, Dennis MS, Silverman AP, Zuchero YJ. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. 2020 May 27;12(545) PubMed.
- Ullman JC, Arguello A, Getz JA, Bhalla A, Mahon CS, Wang J, Giese T, Bedard C, Kim DJ, Blumenfeld JR, Liang N, Ravi R, Nugent AA, Davis SS, Ha C, Duque J, Tran HL, Wells RC, Lianoglou S, Daryani VM, Kwan W, Solanoy H, Nguyen H, Earr T, Dugas JC, Tuck MD, Harvey JL, Reyzer ML, Caprioli RM, Hall S, Poda S, Sanchez PE, Dennis MS, Gunasekaran K, Srivastava A, Sandmann T, Henne KR, Thorne RG, Di Paolo G, Astarita G, Diaz D, Silverman AP, Watts RJ, Sweeney ZK, Kariolis MS, Henry AG. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci Transl Med. 2020 May 27;12(545) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University Kiel
This is an elegant study by Ullman and colleagues of the Denali group where the proof of concept of a therapeutic approach is provided to treat the neurological symptoms in Hunter disease (iduronate-2-sulfatase (IDS) deficiency). The study employs a novel type of fusion protein to overcome the blood-brain barrier, which can hardly be passed by “classical“ enzyme replacement therapies (ERT), although exceptions for the successful CNS corrections by ERT have been described (e.g. Stroobants et al., 2017; Damme et al., 2015; Damme et al., 2011). This is also the case when IDS is intravenously applied to an IDS knock-in mouse model. The enzyme does not reach the brain where it is urgently needed.
To overcome this problem the authors used a newly developed ETV:IDS protein, which represents a fusion of the lysosomal IDS to an Fc domain modulated to interact with the transferrin receptor. This fusion protein turned out to be extremely successful in reaching the brain and all affected cells, including microglia. Most importantly the treatment with rather high doses (40mg/kg) led to a correcting of lysosomal storage and improved the neurological phenotype, i.e., neuroinflammation, neuroaxonal injury, and neurodegeneration. This is certainly a major step forward in the development of an effective therapy of Hunter disease (MPSII) and maybe other lysosomal-storage disorders. It may well be suited for other lysosome-related neurological diseases.
However, a word of caution may be justified. The fusion protein, which requires regular dosing, is unknown to the body and may well cause unwanted immunological reactions and the generation of antibodies, which could affect the long-term efficacy of such a treatment protocol. Also, blocking the transferrin receptor with the therapeutic fusion enzyme may cause unexpected long-term problems. It will be interesting to learn more about the intracellular delivery routes and fate of ETV:IDS proteins. It should also be mentioned that in 2018 a similar study of a blood-brain-barrier-penetrating fusion protein consisting of an anti-human transferrin receptor (hTfR) antibody and intact hIDS was shown to be successful in mice and monkeys (Sonoda et al., 2018).
Despite the above-mentioned questions, I am convinced that this, or a modified treatment regime, may be exploited for the preclinical and later clinical treatment of a variety of neurological disorders.
References:
Stroobants S, Damme M, Van der Jeugd A, Vermaercke B, Andersson C, Fogh J, Saftig P, Blanz J, D'Hooge R. Long-term enzyme replacement therapy improves neurocognitive functioning and hippocampal synaptic plasticity in immune-tolerant alpha-mannosidosis mice. Neurobiol Dis. 2017 Oct;106:255-268. Epub 2017 Jul 15 PubMed.
Damme M, Stroobants S, Lüdemann M, Rothaug M, Lüllmann-Rauch R, Beck HC, Ericsson A, Andersson C, Fogh J, D'Hooge R, Saftig P, Blanz J. Chronic enzyme replacement therapy ameliorates neuropathology in alpha-mannosidosis mice. Ann Clin Transl Neurol. 2015 Nov;2(11):987-1001. Epub 2015 Sep 19 PubMed.
Damme M, Stroobants S, Walkley SU, Lüllmann-Rauch R, D'Hooge R, Fogh J, Saftig P, Lübke T, Blanz J. Cerebellar alterations and gait defects as therapeutic outcome measures for enzyme replacement therapy in α-mannosidosis. J Neuropathol Exp Neurol. 2011 Jan;70(1):83-94. PubMed.
Sonoda H, Morimoto H, Yoden E, Koshimura Y, Kinoshita M, Golovina G, Takagi H, Yamamoto R, Minami K, Mizoguchi A, Tachibana K, Hirato T, Takahashi K. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol Ther. 2018 May 2;26(5):1366-1374. Epub 2018 Mar 6 PubMed.
University of Southern California
This is an elegant and comprehensive study that describes development of an engineered Fc fragment (called a transport vehicle, TV) that binds to the apical domain of the human transferrin receptor (TfR) at the blood-brain barrier (BBB) at a TfR site that is distinct from its binding sites for transferrin, a natural ligand for TfR and FcRn. Using anti-BACE1 antibodies bound to their TV increased substantially brain uptake of the antibody and effectively reduced endogenous Aβ levels in mice and nonhuman primates.
Brain capillary TfR was proposed years ago by Bill Pardridge as an excellent candidate target for delivery across the BBBs of mice and nonhuman primates of neuroactive proteins and peptide biologics, for example growth factors, when they are linked to anti-TfR antibodies. The new platform described in the current STM paper advances importantly this approach by allowing for numerous additional configurations, including bispecific antibodies and protein fusions, that could be delivered to high concentrations in the CNS using the novel, highly modular CNS delivery platform.
Some questions in the field, however, persist, such as how the BBB function is compromised in many human neurological diseases, particularly in the regions of therapeutic interest, for example the medial temporal lobe in Alzheimer’s. Whether TfR at the BBB remains functional or not at these affected sites remains to be seen. Thus, site-specific delivery of therapeutics, including proteins, to affected CNS regions could be important for many neurological disorders. Interestingly, some of these issues seem to be at least partially solved by CSF delivery thereby completely circumventing the BBB, as shown for example with designer DNA drug therapy for neurodegenerative disorders.
Uppsala University
BioArctic
Monoclonal antibodies are very interesting as therapeutics, also for Alzheimer’s disease. They can be made highly specific for their target, which is an obvious advantage when focusing on aggregating proteins where not all forms are toxic. However, one problem is the low penetrance of antibodies into the brain. It is usually claimed that about 0.1 percent of the given drug eventually crosses the blood-brain barrier (BBB) and passes into the brain. This is also the case for other biologics like enzymes, which prohibits the use of standard administration of enzyme replacement therapy for neurological disorders.
The papers from the Denali team describe an excellent step in evolving a technology platform using the transferrin receptor (TfR) as a way of crossing the BBB. It is difficult to envision a smaller adjustment to an antibody structure to get it to function as a vehicle to pass the BBB. By randomizing a specific region involving nine amino acids of the Fragment crystallizable (Fc) region, in the CH3 domain, they manage to identify binders to the TfR. This technology was originally developed by the company F-star for making bispecific antibodies (Fcab).
The modified Fc, which Denali named transport vehicle (TV), can ferry different types of biologics over the BBB. The TV binds to the TfR at the apical domain without interfering with the endogenous ligand transferrin. However, recently it was described that another endogenous ligand to TfR, ferritin, also binds to a large epitope on the apical domain (Montemiglio et al., 2019). If the TV overlaps with this epitope on TfR, an obvious question is if this will have consequences, as ferritin serves to store iron in a nontoxic form, and to transport it to areas where it is required. Ultimately only the coming clinical data will inform us about this.
One interesting aspect of the TV’s design is using a monovalent binding mode to TfR by using the knob-into-hole (KiH) technology. It would be interesting understand why this is necessary, as the group has previously only focused on relatively low affinity to TfR to successfully getting across the BBB (Yu et al., 2011).
A group at Roche has developed a similar strategy to deliver biologics to the brain, the Brain Shuttle technology. They argue that the monovalent binding mode is absolutely crucial for an efficient and safe transport by the TfR in order not to interfere with the intracellular trafficking of TfR (Niewoehner et al., 2014). This Brain Shuttle approach is currently in Phase 1 trials for Alzheimer’s disease (RG6102). The gain in brain exposure by using lower affinity binding to TfR is likely due to the longer plasma half-life, as the TV is not rapidly sequestered by cells in the periphery that also express TfR.
A standard bivalent antibody with high affinity to TfR, where the therapeutic is linked in the back of the Fc, could potentially negatively affect the homeostasis of TfR, especially when using frequent dosing. This approach is currently being investigated in Phase 2/3 in the clinic (see trial) for the treatment of Mucopolysaccharidosis type II (MPS II), also known as Hunter syndrome, by the company JCR Pharmaceuticals. Denali will investigate its TV platform in the clinic for the same indication.
Recently it was shown that the Brain Shuttle platform developed by Roche had reduced engagement with Fc gamma receptor (FcγR) when it was bound to the TfR. This could be of importance when using antibody subtypes with effector function, as it attenuates immune responses involving cells expressing TfR in the periphery. Therefore, it would be of importance to investigate if the platform developed by Denali interacts with other endogenous Fc ligands, e.g. FcγR, in its normal mode when it is bound to the TfR.
One obvious question is whether the amino-acid changes made in the Fc part of the antibody will induce immune responses. Immunogenicity can limit the use of biopharmaceuticals, particularly for the treatment of chronic diseases.
Overall, the paper from the Denali group is of high quality and further enhances our knowledge on how to deliver biologics to the brain in a noninvasive manner. It is a beautiful combination of antibody engineering and biological understanding of the BBB that drives this brain delivery platform development.
In the future, with effective platforms for BBB penetration being used for biological drugs, we expect not only less cost of goods, but also better efficacy as these molecules also seem to penetrate better into different compartments of the brain.
References:
Montemiglio LC, Testi C, Ceci P, Falvo E, Pitea M, Savino C, Arcovito A, Peruzzi G, Baiocco P, Mancia F, Boffi A, des Georges A, Vallone B. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat Commun. 2019 Mar 8;10(1):1121. PubMed.
Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, Dennis MS. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011 May 25;3(84):84ra44. PubMed.
Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, Loetscher H, Ghosh A, Freskgård PO. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014 Jan 8;81(1):49-60. PubMed.
Weber F, Bohrmann B, Niewoehner J, Fischer JA, Rueger P, Tiefenthaler G, Moelleken J, Bujotzek A, Brady K, Singer T, Ebeling M, Iglesias A, Freskgård PO. Brain Shuttle Antibody for Alzheimer's Disease with Attenuated Peripheral Effector Function due to an Inverted Binding Mode. Cell Rep. 2018 Jan 2;22(1):149-162. PubMed.
Make a Comment
To make a comment you must login or register.