Human Microglia Eat Synapses, More So in Alzheimer’s
Quick Links
In mice, microglia eat synapses. Does this happen in the human brain, too? New data from the labs of Tara Spires-Jones and Barry McColl, both at the University of Edinburgh, suggests as much. In postmortem samples, the researchers uncovered remnants of synapses inside these brain-resident immune cells. In cell culture, primary human microglia gobbled up synaptic material, more so if it came from the brain of a person with Alzheimer’s disease. The results, posted to bioRχiv October 7, imply that human microglia have synapses on their menu, and that this could be a factor in AD.
- Synapse proteins spotted in the bellies of microglia in postmortem human brain.
- Primary human microglia engulf human synaptic bits, especially from Alzheimer’s brain.
- The results don’t say if this is beneficial or detrimental in AD pathogenesis.
“This is a really nice paper,” wrote Cynthia Lemere, Brigham and Women’s Hospital, Boston, to Alzforum. “It is the first evidence that human microglia ingest synaptic protein.” Lemere was not involved in the study.
“This is very important work,” agreed Susanne Krasemann, University Medical Center Hamburg-Eppendorf. “The authors have taken a necessary step toward our understanding of human microglia phagocytic function in aging and disease.”
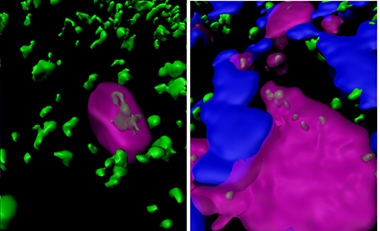
Munching Microglia. In postmortem Alzheimer’s tissue, synapsin I (green/gray) appears inside brain microglia (magenta), both in areas with and without plaques (blue). [Courtesy of Tzioras et al., 2019.]
Synapse loss correlates closely with cognitive decline in AD (DeKosky and Scheff, 1990; Terry et al., 1991). In mice, microglia eat synapses (Hong et al., 2016). In humans, growing evidence implicates microglia in AD, with microglia becoming activated in the disease and microglia-expressed genes influencing risk for it (Minett et al., 2016; Karch and Goate, 2015). Even so, it has been unclear until now whether microglia eat synapses in the human brain.
To find out, first author Makis Tzioras and colleagues examined autopsied brains from 23 AD patients and 17 age-matched healthy controls, sampling the inferior temporal lobe and the visual cortex. Using immunofluorescence and confocal microscopy, they spotted the presynaptic marker synapsin I (SynI) together with CD68, a marker of microglial lysosomes. This happened in both AD and control brains, regardless of the person’s sex, ApoE status, or brain region. However, in temporal lobe AD tissue, the proteins co-localized much more often, implying that microglia ingest more synapses in AD. This was especially true within a 20 micron halo around plaques, where more microglia congregated, and CD68 co-localized with SynI up to three times more than in healthy tissue (see image above).
To find out what might have attracted microglia to synapses from an AD brain in particular, the group prepared synaptoneurosomes—biochemically isolated synapses that contain pre- and post-synaptic terminals—from frozen AD and control brains. They plated them, labeled them, and added microglia isolated from biopsy tissue of an adult undergoing surgery for epilepsy. Within an hour, about half the microglia had taken up synaptoneurosomes into their lysosomes, from both AD and control brains. More microglia went into phagocytic mode if exposed to AD synapses, and they devoured those synapses about 20 percent faster. The data imply that synapses in the AD brain may be more liable to be eaten by microglia than synapses in a healthy brain.
“The AD synaptoneurosomes may be putting out an ‘eat-me’ signal,” Lemere said. “Identification of such a signal might lead to some new therapeutic targets.” She suggested that electron microscopy of the digested material inside microglia would help confirm it was synaptic.
It’s still unclear whether the microglia ingest only dysfunctional synapses or healthy ones as well, said Spires-Jones. While the thought of one’s brain cells eating one’s synapses might strike one as vaguely cannibalistic, it could be beneficial. More synapses die in AD brain, and more ingestion by microglia is necessary to clear them away, Spires-Jones said. In this case, a therapy wouldn’t want to interfere. However, data from mice suggest that microglia are not so discerning, eating not only dead and dying synapses, but healthy, functional ones, as well (Jul 2019 conference news).
For her part, Krasemann is curious about how closely the cultured human microglia resemble those in the brain. Tzioras said that morphologically, they looked normal. While the microglia appear amoeboid just after surgery, they re-extended their processes again during an eight-day recovery culture period before the synapse-eating experiments. Next, the researchers will compare the RNA output of these microglia to those freshly isolated from human brains to make sure the two are transcriptionally similar and enriched in microglial genes, Tzioras said (Galatro et al., 2017).
The postmortem brains in this study all came from end-stage AD. “It would be interesting to know if the non-demented control cases that had some AD pathology had increased Syn1/CD68 co-localization in the brain regions with early Aβ pathology,” Lemere said.
“It is very exciting to see evidence of synaptic engulfment by microglia in human AD, which has been elusive until now,” said Renzo Mancuso, KU Leuven, Belgium, a postdoctoral researcher in the lab of Bart De Strooper. These scientists reported in the October 28 Nature Neuroscience that human microglia can be introduced into the mouse brain, where they survive, integrate, and express genes matching those from human microglia freshly isolated from brain. This includes 44 AD-risk genes, 15 of which have no adequate orthologs in mice. Alzforum covered these findings at AD/PD (Apr 2019 conference news).
“Our [chimeric] model provides a unique platform to study the interaction of human microglia and synapses in the context of the AD brain, and will open new avenues as to the particular molecular mechanisms underlying this interaction in both the synaptic or microglial compartments,” Mancuso wrote to Alzforum. His experiments showed that cultured human microglia were artificially activated, prompting him to wonder whether the microglia from the current paper were, as well.
Spires-Jones sees both models as complementing one another. “The chimeric mouse system is useful for looking at human microglia in a living, aging brain with AD-like pathology,” she wrote to Alzforum. However, it has mouse neurons and synapses, and is less useful for testing potential pathways or therapeutics. On the other hand, while her culture system is easy to manipulate and suitable for quick mechanistic studies, it lacks the full complement of brain cell types that influence microglial function. “Multiple approaches are essential for working toward therapeutically useful results,” Spires-Jones said.—Gwyneth Dickey Zakaib
References
News Citations
- Do Microglia Finish Off Stressed Neurons Before Their Time?
- Chimeric Mice: Can They Model Human Microglial Responses?
Paper Citations
- Dekosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990 May;27(5):457-64. PubMed.
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572-80. PubMed.
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016 May 6;352(6286):712-6. Epub 2016 Mar 31 PubMed.
- Minett T, Classey J, Matthews FE, Fahrenhold M, Taga M, Brayne C, Ince PG, Nicoll JA, Boche D, MRC CFAS. Microglial immunophenotype in dementia with Alzheimer's pathology. J Neuroinflammation. 2016 Jun 2;13(1):135. PubMed.
- Karch CM, Goate AM. Alzheimer's Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biol Psychiatry. 2015 Jan 1;77(1):43-51. Epub 2014 May 17 PubMed.
- Galatro TF, Holtman IR, Lerario AM, Vainchtein ID, Brouwer N, Sola PR, Veras MM, Pereira TF, Leite RE, Möller T, Wes PD, Sogayar MC, Laman JD, den Dunnen W, Pasqualucci CA, Oba-Shinjo SM, Boddeke EW, Marie SK, Eggen BJ. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci. 2017 Aug;20(8):1162-1171. Epub 2017 Jul 3 PubMed.
Further Reading
Papers
- Tzioras M, Daniels MJ, King D, Popovic K, Holloway RK, Stevenson AJ, Tulloch J, Kandasamy J, Sokol D, Latta C, Rose J, Smith C, Miron VE, Henstridge C, McColl BW, Spires-Jones TL. Altered synaptic ingestion by human microglia in Alzheimer’s disease. BioRxiv, October 7, 2019. BioRxiv.
- Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, Srinivasan K, Huntley MA, Wang Y, Wang TM, Hedehus M, Barck KH, Stark M, Ngu H, Foreman O, Meilandt WJ, Elstrott J, Chang MC, Hansen DV, Carano RA, Sheng M, Hanson JE. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019 Aug 20;28(8):2111-2123.e6. PubMed.
- Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, Stevens B, Lemere CA. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017 May 31;9(392) PubMed.
Primary Papers
- Tzioras M, Daniels MJ, King D, Popovic K, Holloway RK, Stevenson AJ, Tulloch J, Kandasamy J, Sokol D, Latta C, Rose J, Smith C, Miron VE, Henstridge C, McColl BW, Spires-Jones TL. Altered synaptic ingestion by human microglia in Alzheimer’s disease. BioRxiv, October 7, 2019. BioRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.