Hot in Here? Or Is it Just My Mitochondria?
Quick Links
Mitochondria sizzle at more than 10°C above body temperature when respiration is running full-tilt, according to a paper in the January 25 PLOS Biology. A new fluorescent sensor enabled researchers led by Pierre Rustin, Inserm, Hôpital Robert Debré, Paris, to record the heat in these organelles in human cultured cells.
“When we investigate the molecular physiology of cells we consider pH, oxidative stress, energy levels, but most of us don’t consider temperature. It could be just as fundamental,” said Russell Swerdlow, University of Kansas, Kansas City. George Perry University of Texas, San Antonio, agreed. “This is very exciting, if correct. It changes everything in terms of mitochondrial function.”
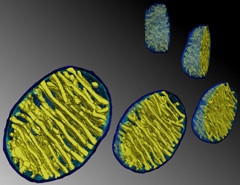
Rounded Radiators.
Temperatures in mitochondria can soar to close to 50°C, perhaps in part due to their parallel membrane arrays. [Courtesy of Terrence G. Frey, PLOS One, 2009.]
Previous studies suggested mitochondria run hotter than other cellular compartments (see review, Sakaguchi et al., 2015). However, some scientists have questioned the plausibility of large temperature gradients within cells, and suspected that findings might be confounded by probes responding to factors besides temperature (Baffou et al., 2014).
To skirt this pitfall, first author Dominique Chrétien used the temperature sensor MitoThermo Yellow. MTY localizes to the organelles. Its fluorescence drops as temperature rises but is unfazed by changes in pH, viscosity, metal ions, oxygen levels, and reactive oxygen species (Arai et al., 2015). Chrétien treated HEK293 human embryonic kidney cells and primary skin fibroblasts with 100 nM MTY, washed away excess probe after 10 minutes, and then measured MTY fluorescence.
When the cells were first oxygen-deprived and then given oxygen-rich media to trigger respiration, MTY fluorescence fell, indicating the mitochondria warmed up by 7–12°C from their prior temperature of 38°C within 20 minutes. The organelles stayed cool, however, when their DNA was depleted by ethidium bromide, or when exposed to respiration inhibitors. The researchers also found that several enzymes of the respiratory chain worked most efficiently at, or slightly above, 50°C, but only when the mitochondrial membranes were intact. “These findings strongly suggest a vital role for the integrity of the inner mitochondrial membrane structure in the stabilization of respiratory chain complexes at high temperature,” wrote the authors.
This 10°C difference “challenges many of our cherished beliefs,” wrote Nick Lane, University College London, in an accompanying commentary. Indeed, some calculations suggest it is physically impossible. “But are these calculations correct?" asked Lane. Probably not, because they are based on simplified models of mitochondria, he writes. For example, mitochondria are more like radiators, with narrow zones sandwiched between heat-producing membranes (see image above), than the simple spheres used in models.
What does this mean for neurons in health and disease? “It’s much too early to say, but it might be quite important,” noted Rustin, who is generating neurons from induced pluripotent stem cells to take their mitochondrial temperature. Swerdlow agreed. “There might be hotspots in neurons, possibly at synapses, where mitochondria are densely packed. If the fire burns too hot, could it singe the synapse?” Running too cool, perhaps as mitochondria lose efficiency with age, could spell trouble as well. Mitochondrial uncouplers, which cause respiration to accelerate, are purported to increase longevity in mice. Uncoupling proteins can have profound effects on neuron function (Caldeira da Silva et al., 2008; Andrews et al., 2005). Swerdlow also wondered if mitochondrial heat might affect local protein folding by altering protein shape directly or by inducing stress responses, such as the heat-shock response.—Marina Chicurel
References
Paper Citations
- Sakaguchi R, Kiyonaka S, Mori Y. Fluorescent sensors reveal subcellular thermal changes. Curr Opin Biotechnol. 2015 Feb;31:57-64. Epub 2014 Aug 29 PubMed.
- Baffou G, Rigneault H, Marguet D, Jullien L. A critique of methods for temperature imaging in single cells. Nat Methods. 2014 Sep;11(9):899-901. PubMed.
- Arai S, Suzuki M, Park SJ, Yoo JS, Wang L, Kang NY, Ha HH, Chang YT. Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem Commun (Camb). 2015 May 11;51(38):8044-7. Epub 2015 Apr 13 PubMed.
- Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008 Aug;7(4):552-60. Epub 2008 Jul 10 PubMed.
Further Reading
Papers
- Nakano M, Arai Y, Kotera I, Okabe K, Kamei Y, Nagai T. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS One. 2017;12(2):e0172344. Epub 2017 Feb 17 PubMed.
Primary Papers
- Chrétien D, Bénit P, Ha HH, Keipert S, El-Khoury R, Chang YT, Jastroch M, Jacobs HT, Rustin P, Rak M. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 2018 Jan;16(1):e2003992. Epub 2018 Jan 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Kansas
This is an interesting observation and the thermos-sensitive fluorescent probe may become a tool for observing mitochondrial function. Based on the results generated from in vitro human cell lines, it appears that mitochondrial respiratory function positively associates with in situ temperature. This needs to be validated by other methodologies independent of this Flu-probe and in multiple cells including primary neuronal cells, since neurons are most demanding for mitochondrial energy and ATP production. It could be more important to validate the physiological role of high temperature in in vivo models. Additionally, the biological significance from this study is unclear and requires further investigation.
Chemically, it is unclear what properties of this Flu-probe make it resistant to temperature and accurate for monitoring temperature in the in vivo setting and during pathophysiological conditions.
Make a Comment
To make a comment you must login or register.