Herpes Simplex Virus Triggers Amyloidosis in 3D Neural Cultures
Quick Links
Looking for a way to model the human brain in a dish? Consider using a gel-filled silk sponge as a three-dimensional scaffold to mimic the vaunted organ’s architecture. That, at least, is the approach published by David Kaplan at Tufts University in Medford, Massachusetts, and colleagues. The researchers seeded the sponges with human neural stem cells, which differentiated into neurons and astrocytes, formed electrically active connections, and survived for two years. In the May 6 Science Advances, the scientists reported that herpes simplex virus type I (HSV-1) triggered rapid amyloidosis and inflammation in these brain-like gel matrices. Paired helical filaments of tau formed, electrical activity waned, and neurons died. The culture system could be useful for studying the effects of numerous genetic and environmental factors that might lead to AD, Kaplan suggested. “This is a new tool for the field,” he told Alzforum.
- Stable 3D tissue culture proposed as new model for AD pathogenesis.
- In this vaguely brain-like tissue, HSV-1 triggers amyloid and tau pathology.
- Virus boosts inflammatory responses and suppresses synaptic transmission.
Ruth Itzhaki, professor emerita at the University of Manchester, U.K., agreed. “I think it’s going to be useful for future work, and it substantiates previous work relating HSV-1 to Alzheimer’s disease,” she said. Itzhaki noted that the model does not require the use of a familial AD mutation, unlike most existing cellular and animal models of the disease. Other commentators were not so sure, telling Alzforum that this model was poorly characterized and unlike cerebral organoids being developed in other labs.
An earlier three-dimensional cell culture model of Alzheimer’s used a gel matrix to grow human neurons that overexpressed APP with familial AD mutations. Developed by Doo Yeon Kim and Rudolph Tanzi at Massachusetts General Hospital in Charlestown, these cultures formed structures resembling amyloid plaques and tau tangles over six weeks (Oct 2014 news). Adding HSV-1 to these cultures hastened pathology, with plaques popping up after two days (Jun 2018 news).
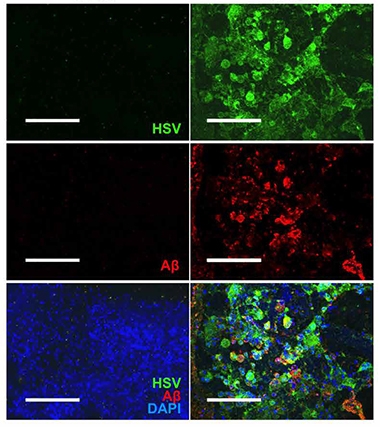
Viral Infection Begets Plaque? After infection with herpes virus (green), amyloid deposits (red) form in three-dimensional neural cultures (right), but not in uninfected cultures (left, nuclei are blue). [Courtesy of Cairns et al., Science Advances.]
Because most cases of AD are sporadic rather than familial, Kaplan wanted to find out if viruses could spark its pathology in human cells from healthy donors. To test this, first author Dana Cairns added HSV-1 to monolayer cultures grown from human neural stem cells. Generated by reprogramming skin fibroblasts, these cells differentiate after four days in culture, with about 90 percent expressing neuronal markers and 10 percent expressing GFAP, a marker of reactive astrocytes (Cairns et al., 2016).
On day four in culture, Cairns added HSV-1 at a concentration of one viral particle per 10,000 cells. Three days later, nearly all the cells contained the virus, and the cultures looked different. They contained large clumps of fused cells, which stained with thioflavin T and bound the anti-tau antibody AT8, suggesting the presence of fibrillar amyloid and paired helical filaments of tau. Infected cultures also secreted about 50 percent more Aβ42 than did uninfected ones. When the researchers added the antiviral valacyclovir at the same time as HSV-1, it prevented formation of these plaque-like structures.

Doughnut Cultures. Cylindrical silk sponges filled with gel allow neurons to grow and to extend axons, supporting formation of functional electrical connections. [Courtesy of Cairns et al., Science Advances.]
Next, the authors turned to their three-dimensional culture model. These grow on doughnut-shaped silk sponges that are 6 millimeters in diameter, with a 2-millimeter hole in the center. After seeding each with human neural stem cells generated from fibroblasts, the authors infused the structure with type I collagen to support neurite extensions, then allowed the cells to grow for four weeks. As in the monolayer cultures, most of the cells are neurons, sprinkled with a few astrocytes. The researchers then added HSV-1 and examined the cultures one week later. As in the monolayers, plaque-like structures had formed that were positive for thioflavin T and also bound the pan-tau antibody tau-1.
Infected cultures appeared inflamed, with soaring expression of GFAP and the inflammatory cytokine TNFα. Expression of numerous AD-related genes changed, as well. Levels of APP fell by two-thirds, while the secretase BACE1 dropped by 80 percent. Loss of APP has been seen after HSV-1 infection before, but the drop in BACE1 was a surprise, the authors noted (Wozniak et al., 2007). Meanwhile, expression of presenilin 1 and 2 increased four- to eight-fold.
Forty other AD genes were massively upregulated, including a 19-fold increase in BACE2 and a fivefold increase in the tau kinase GSK3b. The authors speculated that BACE2 upregulation may compensate for lack of BACE1. BACE2 was recently proposed to protect against amyloidosis (Feb 2020 news). In addition to these expression changes, the authors recorded a drop in electrical activity and saw regions of neuronal loss in the sponge cultures.
Researchers in the field wanted to see further characterization of these three-dimensional cultures, including evaluation of the differentiation state of the cells and the ratio of neurons to astrocytes. They considered the analysis of amyloid and tau aggregates to be inadequate to the field’s standards, and suggested that additional antibodies, dyes, and high-resolution imaging could convincingly demonstrate the presence of fibrillar structures. Some thought the amyloid might not be the same as that seen in AD.
Some commentators spoke only off the record, but Kim, Tanzi, and William Eimer at MGH proposed that rather than resulting from elevated Aβ production, the aggregates might represent an antimicrobial response to the virus, in which the peptide cocoons the pathogen (May 2016 news). “The new three-dimensional model provides further evidence for a role of Aβ as an antimicrobial peptide, under more physiological conditions, free of Aβ overexpression,” they wrote to Alzforum (full comment below).
For his part, Kaplan noted that one advantage of these three-dimensional cultures is their compartmentalized structure, which allows cells to be easily added, tracked, and imaged. In addition, because the cultures survive for longer than two years without necrosis (Rouleau et al., 2020), they could be useful for long-term studies of slowly developing diseases and chronic interventions. In future studies, Kaplan will investigate the effect of different AD-related genetic variants in this system.
Itzhaki suggested examining the effect of ApoE genotype, because previous studies suggest that ApoE4 carriers mount more robust immune responses against pathogens, leading to a higher tangle load after a brain viral infection (Gale et al., 2014; van Exel et al., 2017; Jul 2019 conference news).—Madolyn Bowman Rogers
References
News Citations
- Alzheimer’s in a Dish? Aβ Stokes Tau Pathology in Third Dimension
- Herpes Triggers Amyloid—Could This Virus Fuel Alzheimer’s?
- Can BACE2 Protect Against Amyloidosis?
- Like a Tiny Spider-Man, Aβ May Fight Infection by Cocooning Microbes
- Going Viral: Alzheimer’s Research at Herpes Conference
Paper Citations
- Cairns DM, Chwalek K, Moore YE, Kelley MR, Abbott RD, Moss S, Kaplan DL. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem Cell Reports. 2016 Sep 13;7(3):557-570. Epub 2016 Aug 25 PubMed.
- Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett. 2007 Dec 18;429(2-3):95-100. PubMed.
- Rouleau N, Cantley WL, Liaudanskaya V, Berk A, Du C, Rusk W, Peirent E, Koester C, Nieland TJ, Kaplan DL. A Long-Living Bioengineered Neural Tissue Platform to Study Neurodegeneration. Macromol Biosci. 2020 Mar;20(3):e2000004. Epub 2020 Feb 17 PubMed.
- Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, Azzam KM, Lai L, Blackshear PJ, Calvano SE, Barnes KC, Lowry SF, Corbett S, Wurfel MM, Fessler MB. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014 Jul;134(1):127-34. Epub 2014 Mar 18 PubMed.
- van Exel E, Koopman JJ, Bodegom DV, Meij JJ, Knijff P, Ziem JB, Finch CE, Westendorp RG. Effect of APOE ε4 allele on survival and fertility in an adverse environment. PLoS One. 2017;12(7):e0179497. Epub 2017 Jul 6 PubMed.
External Citations
Further Reading
News
- Invading Microglia Unleash Neurodegeneration in 3D AD Culture
- It Bleeds! New Mini-Brains Sport Functioning Blood Vessels
- Reproducible Brain Organoids Could Offer New Models for Research
- Viral Vectors Trigger Robust Tauopathy in Brain Slices
- Brain Spheroids Hatch Mature Astrocytes
- Mini Brain in a Dish Models Human Development
Primary Papers
- Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv. 2020 May;6(19):eaay8828. Epub 2020 May 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Massachusetts General Hospital, Harvard
Massachusetts General Hospital
Massachusetts General Hospital
Using a new, three-dimensional bioengineered human brain model, Cairns et al. report that HSV-1 infection induces pathological changes reminiscent of those observed in Alzheimer’s disease. This study is consistent with our previous publication, which showed that HSV-1 infection accelerates AD pathology by directly promoting Aβ aggregation in mice and in a three-dimensional human neural cell culture model of AD (Choi et al., 2014; Eimer et al., 2018). In the new study, Cairns et al. show that HSV-1 infection induces multiple AD-like pathological features including multicellular, dense Aβ fibrillar plaque-like formations (PLFs), phospho-tau accumulation, cellular death, astrogliosis, and pro-inflammatory cytokines. Most importantly, this is achieved in naïve human neural cells devoid of FAD mutations or Aβ overexpression. These data support the role of endogenous Aβ as an antimicrobial peptide, consistent with the Antimicrobial Protection Hypothesis of AD first proposed by our group (Soscia et al., 2010; Kumar et al., 2016; Eimer et al., 2018; Moir et al., 2018).
While the use of naïve neural cells in a three-dimensional model brings us closer to physiological conditions, we would caution that it may be too early to conclude that the three-dimensional naïve human neural cells with acute HSV-1 infection reported here constitute a validated model for “herpes-induced sporadic AD.” First, it is not clear that the multicellular, dense Aβ PLFs in this model represent actual Aβ plaques observed in AD patients. Second, increases in phospho-tau levels cannot be directly translated into evidence for neurofibrillary tangles/paired helical filaments without biochemical and histochemical validation. The new three-dimensional model includes elevated levels of pro-inflammatory cytokines and astrogliosis in the HSV-1 infected cultures, which are proposed to mimic “neuroinflammation” in AD. However, it is important to point out that these three-dimensional cultures do not contain microglia; thus, they do not recapitulate AD-related neuroinflammation. This is in contrast to our previous report of a human triculture (neural-microglial-astrocyte) three-dimensional cell model of AD (Park et al., 2018). Finally, it is important to note that the three-dimensional iPSC-derived neural cells used in this new model are probably fetal in status and may, therefore, not accurately recapitulate adult brain physiology following HSV-1 infection. Yet, it is very interesting that the combination of HSV-1 and naïve neurons were able to engender these pathological features.
Regarding the proposed mechanism of pathogenesis in the new model, the authors appear to favor HSV-1 inducing increased production of Ab42, together with increased levels of presenilin 1 (PS1) mRNA levels following HSV-1 infection. MSD analysis revealed moderate increases in levels of Ab42, but not Ab40, following HSV-1 infection. It is important to point out that increased PSEN1 transcription would not, alone, provide evidence of increased or altered PS1/γ-secretase activity. Importantly, the PS1/γ-secretase complex is composed of multiple subunits including PS1, APH1, PEN2 and Nicastrin. Moreover, APP and BACE1 mRNA levels were significantly decreased, suggesting that Aβ levels should be decreased. Thus, it is unlikely that Aβ accumulation in this model is actually due to effects of HSV1 on Aβ production.
In accordance with our Antimicrobial Protection Hypothesis of AD, we propose that the Aβ accumulations observed in this new three-dimensional model were most likely not solely due to Aβ production, but may have been induced by microbial “seeding” of Aβ into beta-amyloid by HSV-1. This would be consistent with the role of Aβ as an antimicrobial peptide (Kumar et al. 2016; Eimer et al. 2018). In brief, we previously showed that upon binding to microbes, Aβ is rapidly “seeded" to form amyloid fibrils, leading to extracellular traps consisting of Aβ. These traps (amyloid deposits) then immobilize microbes to protect host cells, a classic property of antimicrobial peptides (Kumar et al., 2016; Eimer et al., 2018).
In summary, the pathological features observed in this new three-dimensional cell model are consistent with our previous findings (Eimer et al., 2018), and also suggest that HSV-1 may have the ability to nucleate endogenous Aβ derived from naive human neurons, into Aβ deposits, consistent with the role of Aβ42 as an antimicrobial peptide (Moir et al., 2018). Thus, while the authors chose to focus on HSV-1 effects on Aβ production, the new three-dimensional model provides further evidence for a role of Aβ as an antimicrobial peptide, under more physiological conditions, free of Aβ overexpression, i.e., HSV-1 induces “seeding” of endogenous Aβ (in its role as an antimicrobial peptide) from naive neurons leading to Aβ oligomers/fibrils, phospho-tau, and astrogliosis. As such, these findings have broad implications for the ability of HSV-1 and other microbes to rapidly nucleate beta-amyloid deposition, perhaps even in the presence of only physiological levels of Aβ, further supporting the Antimicrobial Protection Hypothesis of AD (Moir et al., 2018).
References:
Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement. 2018 Dec;14(12):1602-1614. Epub 2018 Oct 9 PubMed.
Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014 Nov 13;515(7526):274-8. Epub 2014 Oct 12 PubMed.
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE, Moir RD. Alzheimer's Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron. 2018 Jul 11;99(1):56-63.e3. PubMed.
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016 May 25;8(340):340ra72. PubMed.
Park J, Wetzel I, Marriott I, Dréau D, D'Avanzo C, Kim DY, Tanzi RE, Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer's disease. Nat Neurosci. 2018 Jul;21(7):941-951. Epub 2018 Jun 27 PubMed.
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010 Mar 3;5(3):e9505. PubMed.
Morinaga Milk Industries, Co. Ltd.
If you add any microbes with β-sheet proteins at their surface (not only virus) in this three-dimensional culture system, you will be able to see Aβ plaque formation within two to four hours. This supports the new Alzheimer's disease concept that this syndrome can be caused by microbiome dysbiosis, and expansion of the microbes across the blood-brain barrier. Plaque formation is simply the evidence of this invasion, as the brain's innate immune system tries to tag and engulf the microbes with antimicrobial peptides, amongst other mechanisms.
Make a Comment
To make a comment you must login or register.